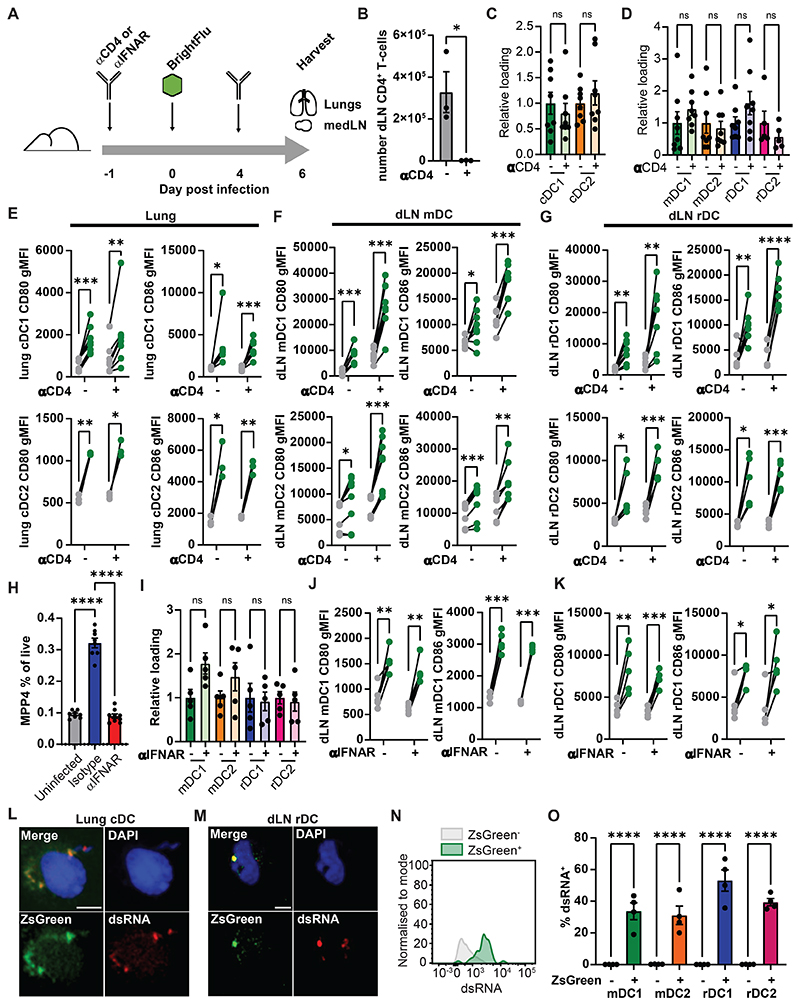
Figure 3. dLN resident cDC are activated independently of CD4+ T cell help and type I IFN signalling.
(A) Schematic showing blocking experiments (B) Quantification of CD4+ T cells in the dLN at day 6 post infection (n=6, 3 experiments) (C) ZsGreen loading of cDC in the lung and (D) dLN at day 6 post infection ± CD4+ T cell depletion (n=16, 3 experiments). (E-G) gMFI for CD80 and CD86 of antigen bearing (green) and non-bearing (grey) cDC subsets ± CD4+ T cell depletion in the lung (E), mDC subsets in the dLN (F) and rDC subsets in the dLN (G) (n=16, 3 experiments). (H) Quantification of haematopoietic multipotent progenitors as a proportion of live cells in the bone marrow of uninfected, isotype and anti-INFRα treated mice (n=25, 3 experiments) (I) ZsGreen relative loading of mDC and rDC subset in the dLN at day 6 post infection ± IFNAR blockade (n=10, 3 experiments)(J-K) gMFI for CD80 and CD86 of antigen bearing (green) and non-bearing (grey) mDC1 (J) and rDC1 (K) in the dLN ± IFNAR blockade (n=10, 3 experiments). (L-M) Confocal images of sorted lung cDC (L) and lymph nodes rDC (M) showing ZsGreen (green), dsRNA (red) and DAPI (blue). Scale bar = 5μm (N) Representative flow of dsRNA staining of ZsGreen+ (green) and ZsGreen- (grey) cDC in the medLN (O) Proportion of ZsGreen+ and ZsGreen- cDC positive for dsRNA staining by flow cytometry(n=4, 2 experiments). Statistical differences were determined by student T test (B, C, D, I), one-way ANOVA with Tukey post-test (H), and two-way ANOVA with Šidák multiple comparison test (E, F, G, J, K, O). P values: ns P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001. Data are shown as mean ± SEM