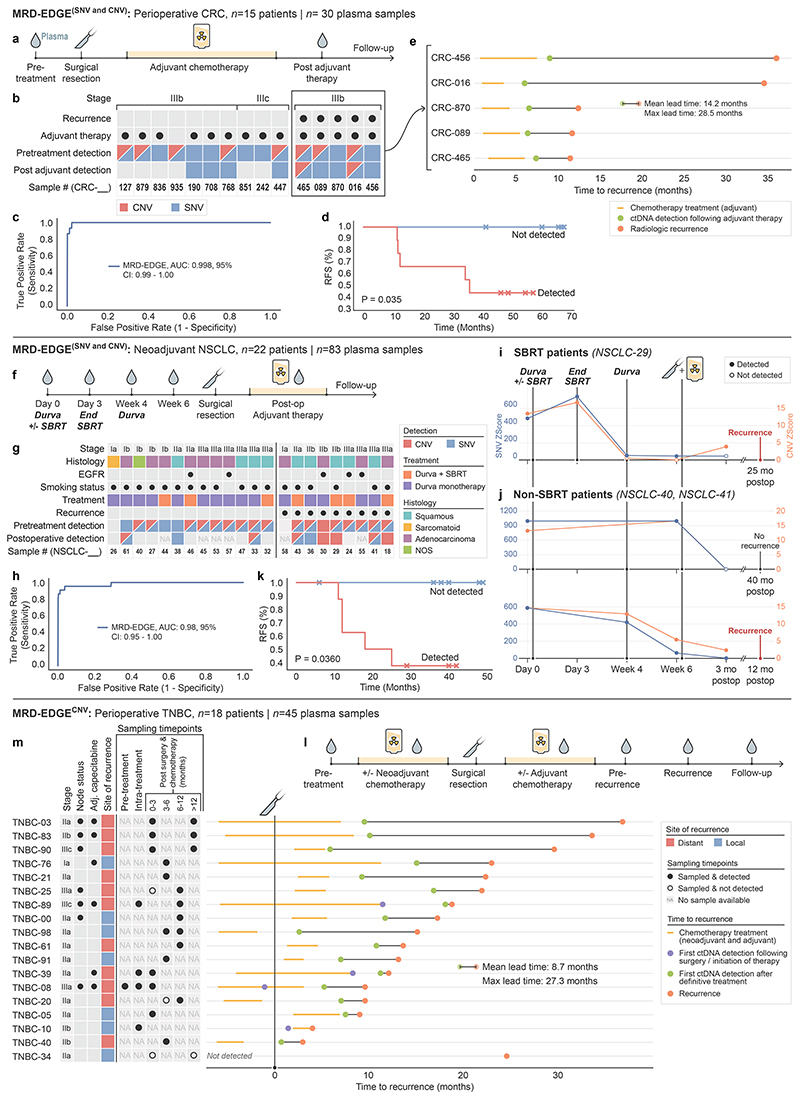
Figure 3. Tumor-informed monitoring of minimal residual disease in perioperative, neoadjuvant, and recurrent disease settings.
a) Perioperative colorectal cancer ctDNA assessment. Plasma TF is tracked prior to surgery, and after surgery and adjuvant chemotherapy. b) Clinical characteristics and detection status of the stage III CRC cohort. c) ROC analysis on MRD-EDGE in preoperative stage III CRC with matched tumor mutation profiles (n=15) compared to control plasma samples assessed against all unmatched stage III CRC tumor mutation profiles (n=15 tumor profiles assessed across 25 control samples from Aarhus controls cohort, n=375 control-comparisons). d) Kaplan–Meier disease-free survival analysis of all patients with detected (n=9) and non-detected (n=6) postoperative ctDNA. Postoperative ctDNA detection was associated with shorter recurrence-free survival (two-sided log-rank test). e) Time to recurrence in stage III CRC patients with disease recurrence (n=5) after ctDNA detection post-therapy (green dot). Red dot indicates confirmed recurrence on CT imaging. f) Neoadjuvant NSCLC clinical treatment protocol41. Plasma TF is tracked in the preoperative period to evaluate for response to SBRT and ICI (durvalumab) therapy and after surgery to evaluate for MRD. g) Clinical characteristics and detection status of the neoadjuvant NSCLC cohort (n=22 patients). h) ROC analysis on MRD-EDGE in pretreatment early-stage NSCLC. Preoperative plasma samples with matched tumor mutation profiles (n=22) are compared with control samples assessed against all unmatched NSCLC tumor mutation profiles (n=22 mutation profiles assessed across 20 control samples from NYGC control cohort, n=440 control-comparisons). i) Tumor burden monitoring on neoadjuvant immunotherapy and SBRT with MRD-EDGESNV (blue) and MRD-EDGECNV (orange) Tumor burden estimates are measured as the Z score of the patient tumor mutation profile against healthy control plasma. j) Tumor burden monitoring with MRD-EDGESNV and MRD-EDGECNV in 2 NSCLC patients on neoadjuvant ICI monotherapy (top, NSCLC-40; bottom, NSCLC-41). Red dot indicates recurrence; black dot indicates absence of recurrence at last known follow-up. k) Kaplan–Meier disease-free survival analysis of all patients with detected (n=8) and non-detected (n=6) postoperative ctDNA. Postoperative ctDNA detection was associated with shorter recurrence-free survival (two-sided log-rank test). i) Observational TNBC recurrence cohort. Early-stage TNBC patients underwent surgical resection plus neoadjuvant and/or adjuvant chemotherapy. Plasma was sampled intermittently throughout clinical course. m) (left) Clinical characteristics and sampling timepoints for the observational TNBC recurrence cohort (n=18 patients). (right) Lead-time calculations for ctDNA detection post-therapy (green dot) versus clinical recurrence (red dot). Where available, purple dot shows ctDNA detection after surgery or initiation of chemotherapy.