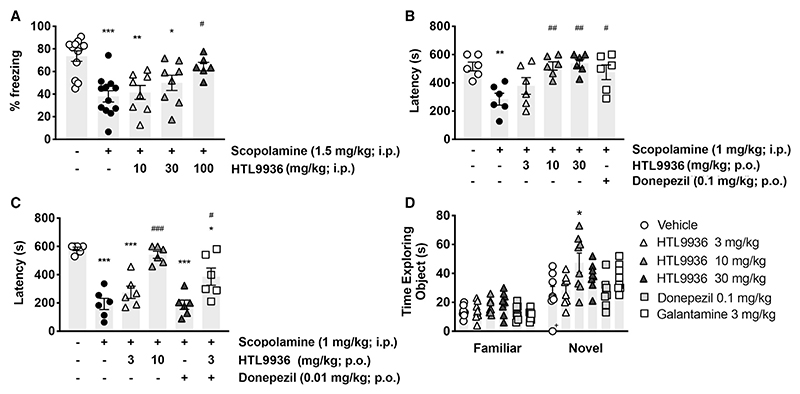
Figure 5. In vivo pharmacological characterization of HTL9936 in rodents.
(A) Effects of HTL9936 (10, 30, or 100 mg/kg; i.p.) on scopolamine (1.5 mg/kg; i.p.)-induced impairments in contextual fear conditioning in male C57BL/6J mice. Data are expressed as means ± SEM of 6−12 mice. Data were analyzed using one-way ANOVA with Bonferroni’s multiple comparison test, where *p < 0.05, **p < 0.01, ***p < 0.001 versus vehicle alone and #p < 0.05 versus 1.5 mg/kg scopolamine-treated.
(B and C) Effects of HTL9936 (3, 10, or 30 mg/kg; p.o.) alone (B) or in combination with donepezil (C) on scopolamine (1 mg/kg; i.p.)-induced amnesia in a passive avoidance paradigm in adult Wistar rats. HTL9936 or donepezil (0.1 or 0.01 mg/kg) were administered 90 min prior to the training period. Data shown are means ± SEM of 6 rats. Data were analyzed using a one-way ANOVA where *p < 0.05, **p < 0.01, ***p < 0.001 versus vehicle alone and #p < 0.05, ##p < 0.01, ###p < 0.001 versus 1 mg/kg scopolamine-treated.
(D) Effects of acute HTL9936 (p.o.) administration on improvement of memory performance in a rodent novel object recognition paradigm. Adult male Wistar rats were treated with vehicle (saline) or HTL9936 (3, 10, or 30 mg/kg) 90 min prior to training. Galanthamine (3 mg/kg) or donepezil (0.1 mg/kg) administered 60 min prior to training were used as positive controls. Time (s) spent exploring the novel object during the testing phase is shown. Data shown are means ± SEM of 8 rats (one animal highlighted with an (+) did not respond in the vehicle group and was removed from the analysis). Data were analyzed using one-way ANOVA with Dunnett’s multiple comparison test, where *p < 0.01 comparing treatment versus vehicle.
See also Figure S6 and Tables S5 and S6.