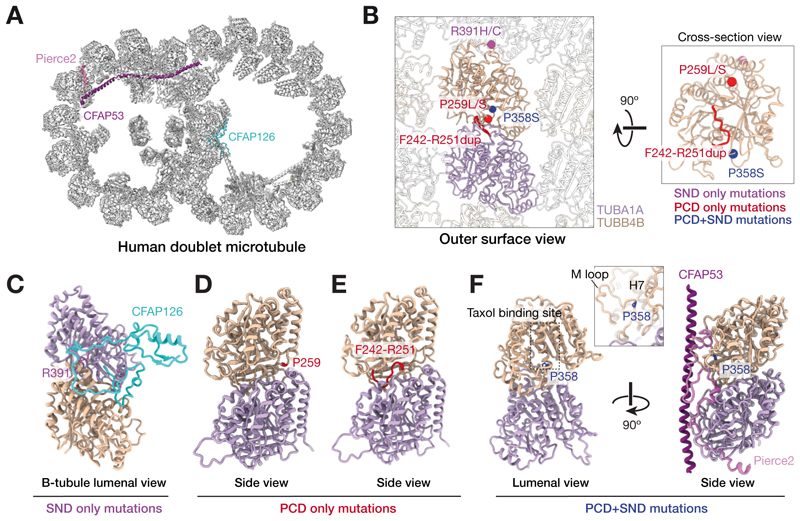
Figure 5. Structural environment of disease-causing variants of TUBB4B.
(A) Human microtubule doublet cross-section (PDB ID: 7UNG) highlighting microtubule interacting proteins (MIPs) that interact with TUBB4B residues associated with disease. Microtubule doublets are the conserved cytoskeletal element of both primary and motile cilia, consisting of complete A-tubule with 13 protofilaments and incomplete B-tubules with 10 protofilaments.
(B) Orthogonal views showing disease-causing TUBB4B variants within the ciliary microtubule doublet lattice. The human tubulin isotypes (TUBA1A (purple) and TUBB4B (gold)) were determined based on the human microtubule doublet cryo-EM density map (32) and abundance in human multiciliated respiratory cells by scRNAseq (64). Variant positions are indicated with spheres colored based on their disease association. Only one TUBB4B molecule is shown in the cross-section (right), where R391 is not visible.
(C) Interaction of R391 of TUBB4B with the microtubule inner protein CFAP126.
(D-E) p.P259 and loop p.F242-R251 locate at the intradimer interface.
(F) p.P358 locates at the taxol binding site which interacts with multiple microtubule interacting proteins (MIPs) including, for example, PIERCE2.