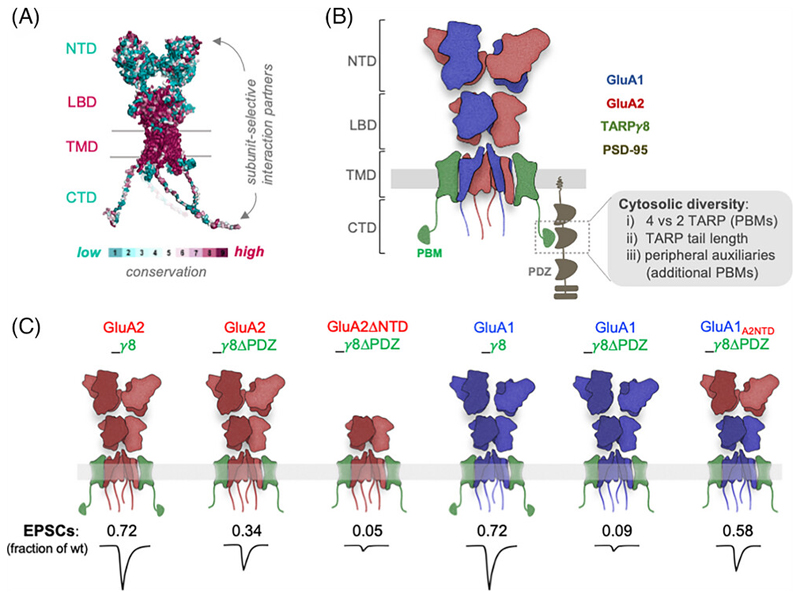
Figure 2.
(A) AMPAR structure colored by sequence conservation between the four subunits (NTD: N terminal domain, LBD: ligand-binding domain, TMD: transmembrane domain, CTD: C terminal domain). The LBD and TMD sequences are highly conserved (magenta), whilst the NTD and CTD show greater sequence diversity (cyan), enabling subunit-specific interactions. (B) Schematic of a heteromeric AMPAR on the postsynaptic membrane, held in the postsynaptic density by interactions between the associated auxiliary protein TARPγ8 and the three PDZ domains of PSD95, and the NTD that extends into the synaptic cleft. Cytosolic diversity of synaptic AMPAR complexes can arise by (i) the stoichiometry of associated TARPs (ii) TARP type, for example, different length C-tails of TARP γ2 and TARPγ8 (iii) PDZ anchoring by additional associated proteins, for example, CKAMPs. (C) Schematic depicting the subunit-specific effect of the NTD and TARPγ8 PDZ interactions on synaptic transmission. Excitatory postsynaptic current (EPSC) amplitude, normalized to a neighboring transfected neuron, is reduced when removing the TARPγ8 PDZ binding motif (γ8ΔPDZ). γ8 PDZ deletion prevents GluA1-mediated synaptic transmission, which can be partially rescued by the NTD of GluA2 (derived from Watson et al., 2021).