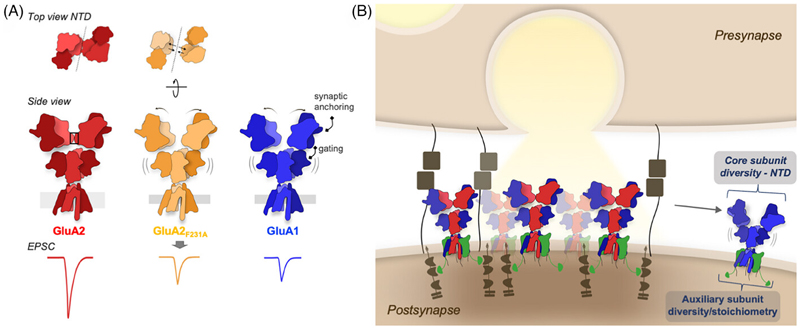
Figure 3.
(A) Proposed model of the role of the NTD in synaptic anchoring of AMPARs. The stability of the tetrameric interface in GluA2-containing receptors (highlighted in black; ‘side view’) may enable efficient synaptic anchoring, as receptors without this interface (GluA1 homomers) or with a disrupted interface (GluA2F231A) show reduced EPSCs following Schaffer collateral stimulation. The flexibility of the NTD with a disrupted interface has implications for both the gating and synaptic anchoring of the receptor. (B) Synaptic accumulation of AMPARs is maintained by TARP (green) PDZ interactions with PSD-95 (brown). Subsynaptic positioning into receptor clusters opposing vesicle release is influenced by both the core subunit, determining subunit-specific NTD interactions, and the associated auxiliary proteins providing PSD-95 anchoring. NTD interactions with synaptic cleft molecules may be disturbed in receptors with a broken NTD dimeric interface, making them less likely to be maintained in a stable synaptic position.