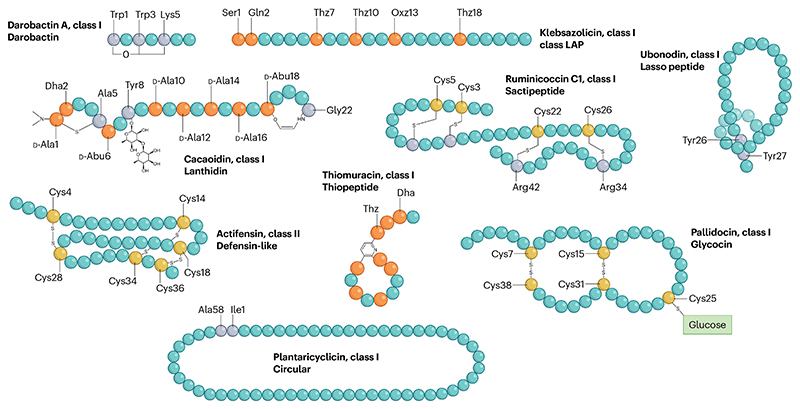
Fig. 1. The structural diversity of bacteriocins.
Bacteriocins are typically classified into classes and subgroups based on their mechanism of production and secondary structures (Table 1). Lanthipeptides and other class I bacteriocins may contain complex intrapeptide bonds that are installed through enzymatic modifications of specific residues in the peptide backbone. These bonds may form small ring structures such as the C–O–C Trp–Trp ether bond in darobactin A (key residues coloured in grey) and the cacaoidin AviMeCys ring (modified residues coloured in orange). Multiple intrapeptide crosslinks may be formed following modification within one peptide such as the sacthioine bonds in ruminicoccin C1 (involved cysteine residues coloured in yellow). Class I bacteriocins may also feature post-translational installation of other moieties, shown here in glycosylated pallidocin at cysteine 25 and tyrosine 8 of cacaoidin. Modified residues are not always part of larger ring structures, such as the thiazole and oxazole residues present in the linear azoline-containing peptide, klebsazolicin. Large ring structures, however, are prominent features of different subgroups of class I bacteriocins and take different forms such as the loop structure present in the lasso peptide ubonodin that is sterically locked by bulky tyrosine side chains or the macrocycle of the heavily modified thiomuracin A. The largest rings are present within the circular bacteriocins wherein N-terminal and C-terminal residues are covalently linked resulting in a closed loop structure. Class II bacteriocins do not undergo enzymatic modification and are therefore less enzymatically decorated but may feature disulfide bonds that can also result in intercalated structures of such as those in defensin-like bacteriocins, such as actifensin. LAP, linear azole-containing or azoline-containing peptide.