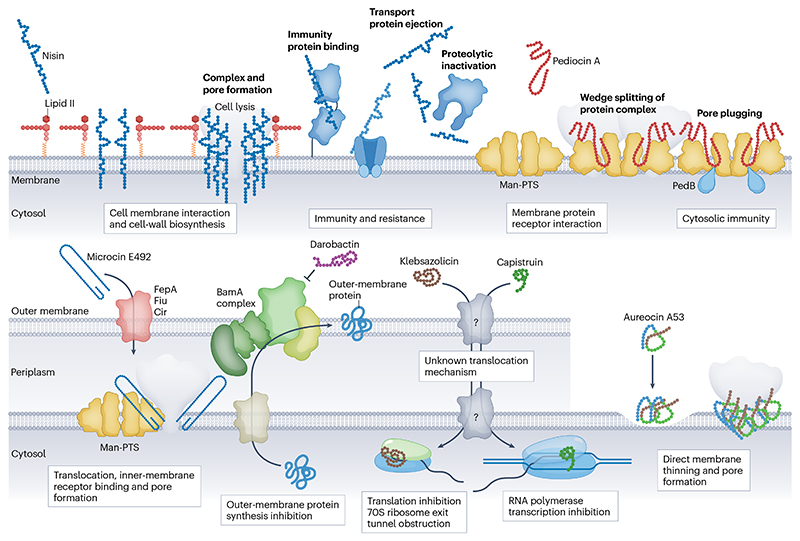
Fig. 2. Bacteriocins mechanisms of action and resistance mechanisms.
Bacteriocins are heterogeneous in function and exhibit various mechanisms of action. Bacteriocins are typically cationic hydrophobic peptides that are drawn towards anionic bacterial cell surfaces. At the surface, they can interact with specific receptors, such as nisin that complexes specifically to the cell-wall precursor molecule lipid II and subsequently forms pores across the membrane, which leads to cell death. Mechanisms of immunity and resistance protect strains from nisin by binding, ejecting or proteolytically cleaving the peptides. Pediocin-like bacteriocins (class II) attach to the extracellular surface of the mannose-phosphotransferase system (Man-PTS) core domain, penetrate the membrane and shift the core domain away from its V-motif, thereby forming a pore in the membrane. PedB proteins in the targeted bacterium can physically plug the pore formed by pediocin PA-1 and the Man-PTS, thereby providing cytosolic immunity. Other peptides target the membrane directly, such as aureocin 53 that initially causes Gram-positive membrane thinning by accumulating in the upper leaflet of the phospholipid bilayer, which is mediated by specific α-helices of the peptide, and then causes poration favoured by other α-helices within the peptide. Bacteriocins can also interrupt essential cellular processes such as outer-membrane protein integration of BamA by darobactin or inhibition of protein and nucleic acid synthesis of klebsazolicin and capistruin, respectively. Microcin E492 translocates across the outer membrane using a ‘Trojan horse’ strategy, wherein the siderophore moiety is recognized by outer-membrane transporters that facilitate uptake of the peptide into the periplasm, followed by binding to the mannose-PTS at the inner membrane and subsequent lethal disruption of membrane potential. Immunity to microcin E492 is provided by the action of the immunity protein MceB, via an unknown mechanism.