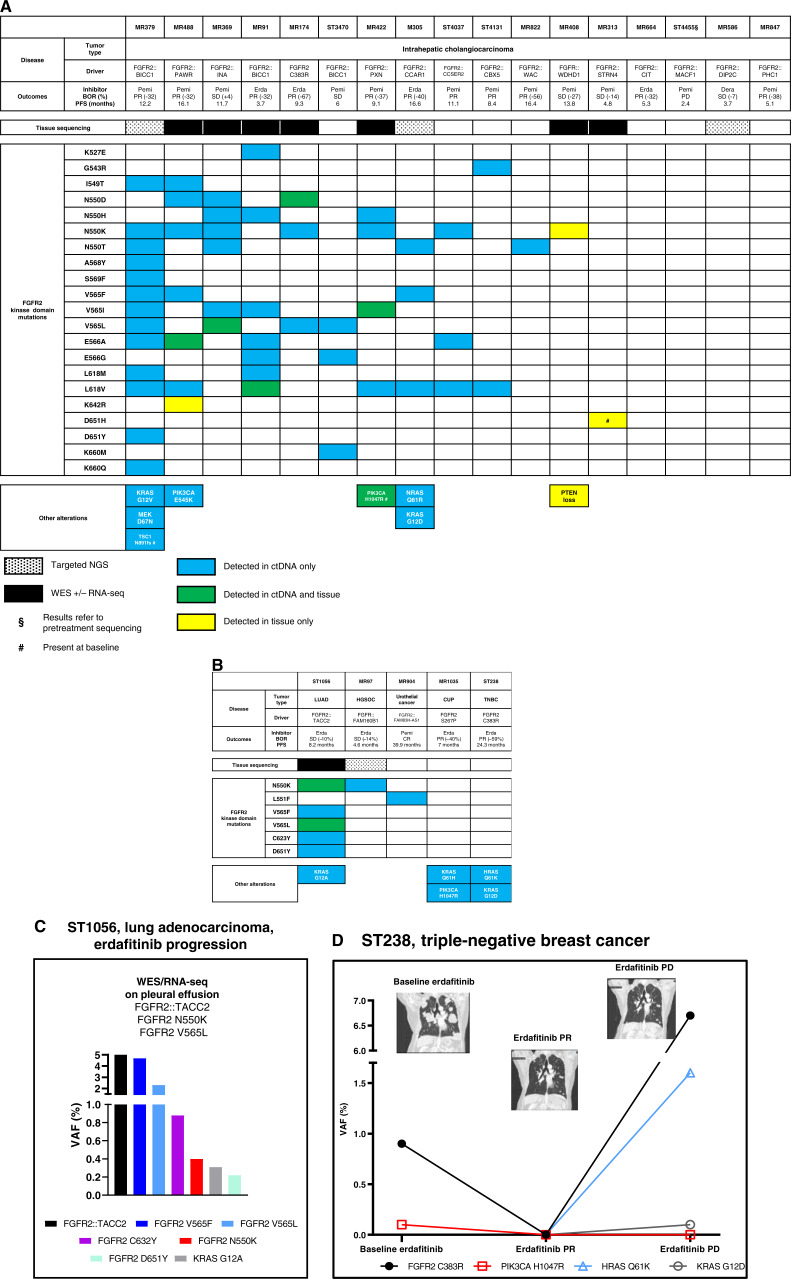
Figure 1.
Molecular findings at resistance to reversible FGFR inhibitors. A, Patients suffering from intrahepatic cholangiocarcinoma. B, Patients suffering from other tumor types. C, Molecular findings of patient ST1056, suffering from a lung adenocarcinoma harboring a FGFR2::TACC2 fusion, at acquired progression to erdafitinib. D, Clinicoradiologic and molecular evolution of patient ST238, suffering from a FGFR2 C383R–driven triple-negative breast cancer. The ctDNA findings are reported, and ctDNA findings are reported as VAF (%). BOR, best objective response; CR, complete response; CUP, cancer of unknown primary; Dera, derazantinib; Erda, erdafitinib; HGSOC, high-grade serous ovarian cancer; LUAD, lung adenocarcinoma; PD, progressive disease; Pemi, pemigatinib; PR, partial response; SD, stable disease; TBNC, triple-negative breast cancer.