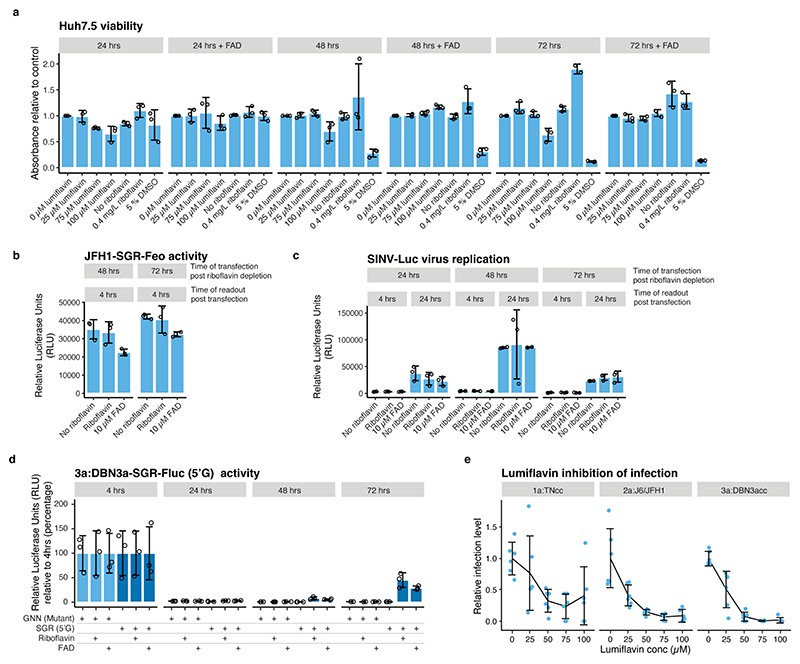
Extended Data Fig.6 |. The effect of riboflavin depletion and lumiflavin treatment on cellular viability and HCV genotype-specific dependency on FAD.
(a) Cell viability after lumiflavin treatment or riboflavin depletion. (b) Measurement of translational activity at 4 hrs after transfection of JFH1-SGR-Feo RNA with and without 0.4 mg/L riboflavin and 10μM FAD. Transfection at several time points after riboflavin depletion was included to exclude that differences in cell viability affected HCV IRES-mediated translation. (c) Measurement of Sindbis virus (SINV) replication after transfection of in vitro transcribed RNA from infectious SINV Toto-1101/Luc reporter virus with and without 0.4 mg/L riboflavin and 10 μM FAD. Transfection at several time points after riboflavin depletion was included to exclude that differences in cell viability affected SINV replication. (d) Replication of DBN3a-SGR(5’G) and GNN (non-replicating mutant) in Huh7.5 cells grown in riboflavin-depleted media. DBN3a-SGR-Fluc(5’G) had partially reverted to 5’A at the 72 hrs time point. Conditions with riboflavin (0.4 mg/L) and FAD (10 μM) included are indicated. Replication was measured in relative luciferase units (RLU) at the indicated time points and quantified relative to 4 hrs. (e) Inhibition of HCV infection by lumiflavin. Infection was measured in focus forming units relative to the untreated control obtained after infection with HCV TNcc (1a), J6/JFH1 (2a) or DBN3acc (3a) strains. For all panels, data are presented as mean +/− SD, n=3 biological replicates.