Table 1. Small molecule inhibitors of disordered states of proteins with proven in vivo efficacy.
| Target | Disease family | Compound | Identification | In vivo efficacy | Clinical trials (Phase) | Structure | Ref. |
|---|---|---|---|---|---|---|---|
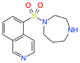
| ɑ-synuclein | Parkinson’s disease | Fasudil | Drug repurposing | Improved motor and cognitive functions at 10 and 30 mg/kg | Approved for use in humans but not assayed for Parkinson’s disease |
|
[37] |
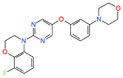
| Aβ | Alzheimer’s disease | SEN1576 | Rational design | Reduced deficits in in vivo long-term potentiation and memory at 0.3 and 1 mg/kg | - |
|
[38] |
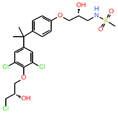
| AR | Castration resistant prostate cancer | EPI-7170 | Second generation lead compound from a phenotypic screen | Tumor growth inhibition at 25 mg/kg | NCT044212221 (1) |
|
[39] |
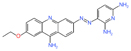
| c-Myc | Cancer | MYCMI-6 | Cell-based protein interaction screen | Apoptosis induction and reduction of tumor cell proliferation and microvascularity at 20 mg/kg | - |
|
[40] |
| EN4 | Covalent ligand screen | Tumor growth inhibition at 50 mg/kg | - |
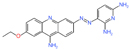
|
[41] | ||
| EWS-FLI1 | Ewing’s sarcoma | TK216 | Rational design | Tumor growth inhibition at 100 mg/kg |
NCT02657005 (1) |
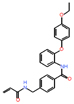
|
[42] |
| NUPR1 | Pancreatic adenocarcinoma | ZZW-115 | Ligand-based design | Tumor growth arrested at a 5 mg/kg | - |
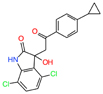
|
[43] |
| PTP1B | Diabetes, obesity | MSI-1436 | Serendipity | Suppresses appetite, reduces body weight and improves plasma insulin at 5-10 mg/kg | Completed: NCT00806338 NCT00606112 NCT00509132 Discontinued: NCT02524951 (1) |
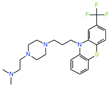
|
[44, 45] |
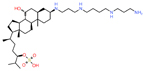
| Tau | Alzheimer’s disease | TRx0237 | In vitro aggregation assay | Reduces the brain atrophy rate at 4 mg/kg twice a day. |
NCT03446001 (1) |
|
[46, 47] |
Clinical trial of EPI-7386 (structure not available).
