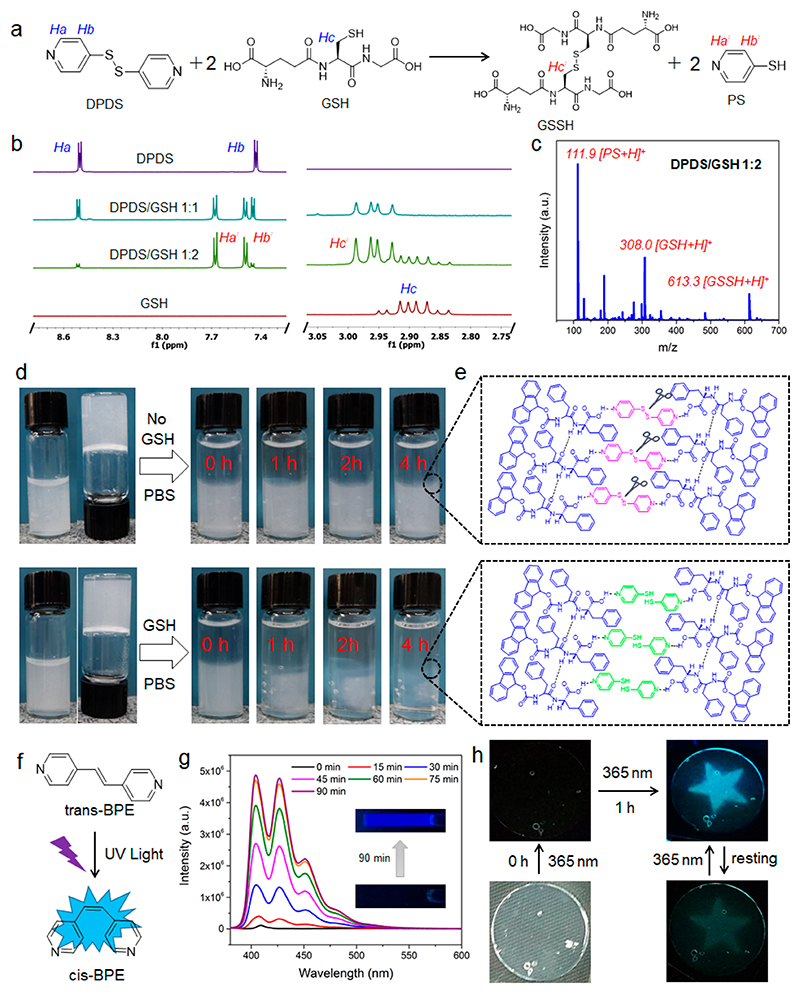
Figure 5.
(a) Proposed chemical reaction of DPDS into PS in the presence of GSH. (b) 1H NMR spectra of DPDS, GSH, and DPDS/GSH mixtures in 1:1 and 1:2 ratios. (c) MS spectrum of 1:2 DPDS/GSH in H2O. The molar concentration of DPDS is 100 μM. (d) Responsiveness of 1:1 FmocFF/DPDS in the absence and presence of GSH (100 mM) in PBS. (e) Proposed assembly and disassembly of FmocFF/DPDS assembly networks before and after cleavage of disulfide bonds by GSH. (f) Photoinduced enhanced emission of BPE after trans−cis transition. (g) Fluorescence emission traces of BPE (50 μM) in water under UV irradiation with varying irradiation durations, from 0 to 90 min. Sample was analyzed after the specified irradiation time by a hand-held UV lamp (6 W) at 365 nm. The insets show fluorescent images of BPE sample before and after UV irradiation for 90 min. The excitation wavelength is 365 nm for all the specified fluorescence emissions. (h) Star-shaped fluorescent imprint of the FmocFF/DPDS gel by 1 h UV irradiation and its self-erasing property following rest in visible light.