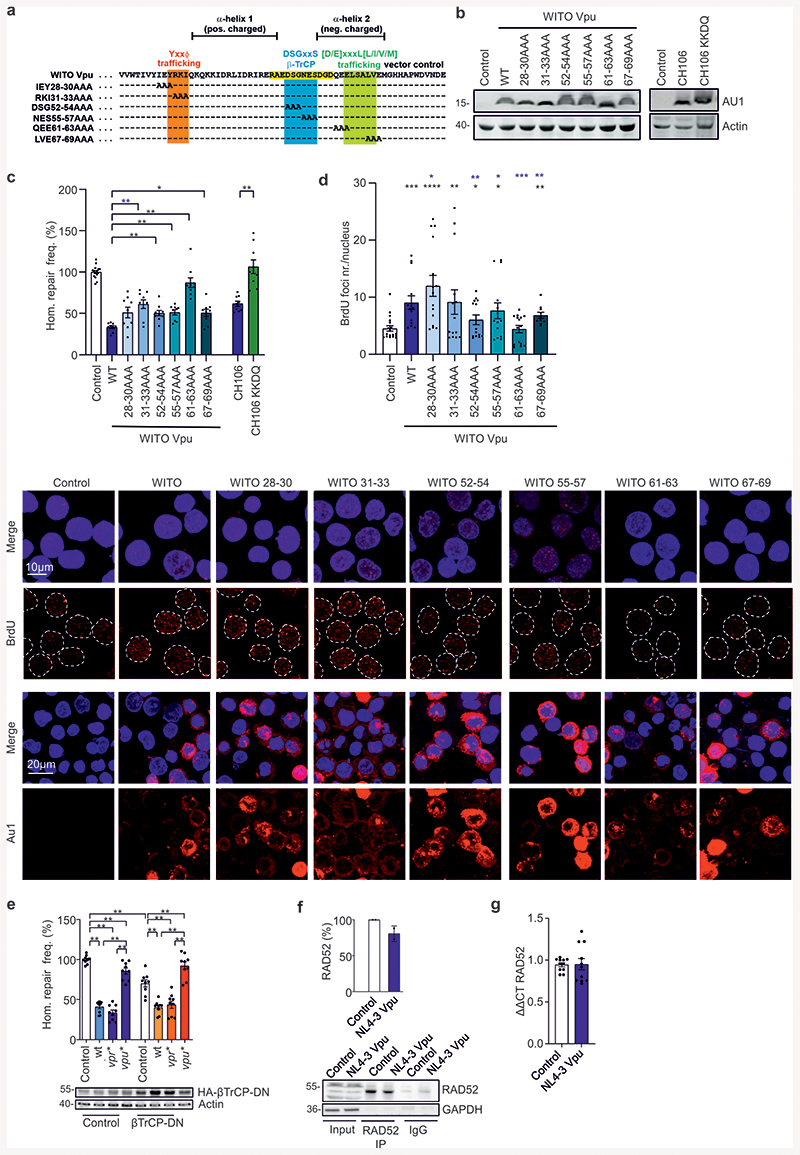
Extended Data Fig. 3. Analysis of mutant Vpu proteins and evaluation of known cellular Vpu targets.
Analysis of mutant Vpu proteins and evaluation of known cellular Vpu targets. (a) Sequence of WITO Vpu with mutated positions indicated. (b) Western blot depicting expression of different WITO Vpu mutants from (a) and CH106 wt and its KKDQ mutant with the ER retention sequence. Experiment repeated 2 times with similar outcome. (c) Homologous repair in WTK1(HR/3’) cells transfected with different mutants of WITO Vpu or CH106 and the CH106 KKDQ mutant with ER retention sequence. 48 h post transfection cells were analyzed by FACS to evaluate DSB repair frequencies. Bars represent means ± SEM, n=3 or 4 biologically independent experiments in triplicates, *p=0.0273, **p=0078, **p=0.0039 (absolute mean value: 0.7 %). Statistical evaluation shown for the comparison between wt WITO Vpu and the indicated Ala substitution mutants. (d) BrdU assay to analyze DNA processing mediated by different WITO Vpu mutants. WTK1(HR/3’) WITO transfected cells were IR (2 Gy), pre-extracted, fixed and stained against BrdU. Data presents 4 independent experiments where each dot (n=14-18) presents mean value of 30-50 analyzed nuclei from one image view, ± SEM. *p=0.0166, **p=0.012, ***p=0.0085, ****p=0.0001. Black stars presenting comparison to the control, blue stars comparison to WITO Vpu. The lower panel shows representative BrdU and AU1 microscopic images. (e) Homologous repair in WTK1(HR/3’) (β-TrCP). Cells were transfected with wt, vpr* or vpu* pHIV-1-NL4-3-env*-IRES-mCherry constructs together with expression plasmids for I-Sce I and the DN form of β-TrCP. 48 h later cells were harvested for FACS analysis. Bars represent means ± SEM, n=3 biologically independent experiments in triplicates, **p=0.039 (absolute mean value: 0.1%). Lower panels show Western blot demonstrating overexpression of β-TrCP-DN. (f) Immunoprecipitation (IP) of RAD52 1h after γ-irradiation (2Gy) of WTK1(HR/3’) cells transfected 24h before with NL4-3 Vpu expression plasmid or empty vector (Control). Graph presents mean values ± SD from two independent experiments. Note that RAD52 is difficult to detect by Western blotting unless it is concentrated e.g. by IP. Thus, most analyses had to rely on mRNA expression levels. (g) Evaluation of RAD52 mRNA levels in SaOS cells treated as described in the legend Fig. 2i. Bars represent means ± SEM, n=4. Two sided Wilcoxon matched-pairs test in c, d, e, g.