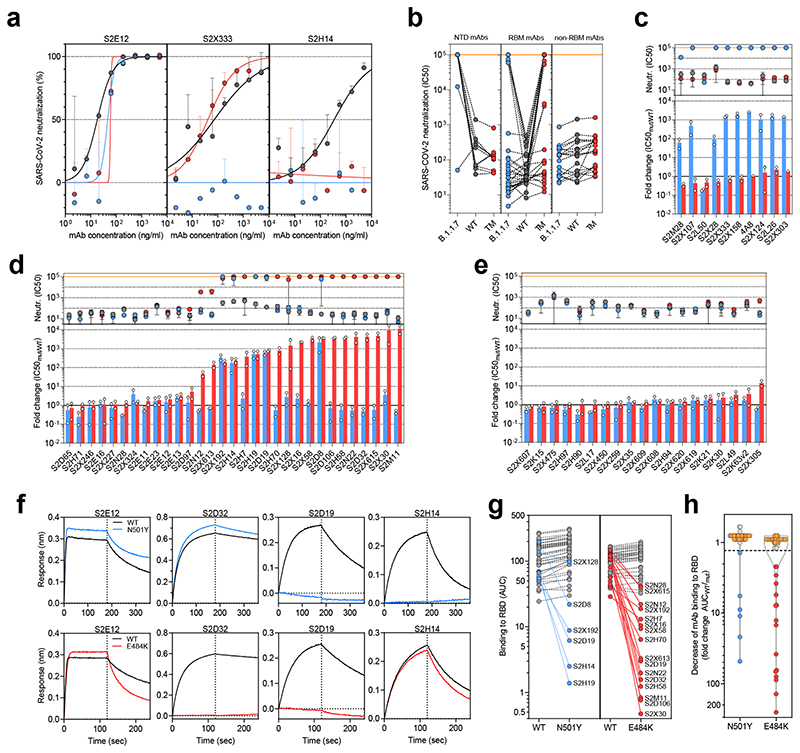
Figure 4. Neutralization and binding by a panel of NTD- and RBD-specific mAbs against WT, B.1.1.7 and RBD mutant SARS-CoV-2 viruses.
a, Neutralization of WT D614G (black), B.1.1.7 (blue) and a triple mutant (TM, carrying RBD mutations K417N/E484K/N501Y) (red) pseudotyped SARS-CoV-2-MLVs by 3 selected mAbs (S2E12, S2X333 and S2H14) from one representative experiment. Shown is the mean ± s.d. of 2 technical replicates. b, Neutralization of WT (D614G), B.1.1.7 and TM SARS-CoV-2-MLVs by 60 mAbs targeting NTD (n=10), RBM (n=31) and non-RBM sites in the RBD (n=19). Shown are the mean IC50 values (ng/ml) of n=2 independent experiments. c-e, Neutralization shown as mean IC50 values (upper panel) and mean fold change of B.1.1.7 (blue) or TM (red) relative to WT (lower panel) of NTD (c), RBM (d) and non-RBM (e) mAbs. Lower panel shows IC50 values from 2 independent experiments. f-h, Kinetics of binding of mAbs to WT (black), N501Y (blue) and E484K (red) RBD as measured by bio-layer interferometry (BLI). Shown in (f) are the 4 RBM-targeting mAbs with no reduced binding to N501Y or E484K RBD. Area under the curve (AUC) (g) and AUC fold change (h) of 50 mAbs tested against WT, N501Y and E484K RBD. mAbs with a >1.3 AUC fold change shown in blue and red. mAbs: monoclonal antibodies. NTD: N-terminal domain