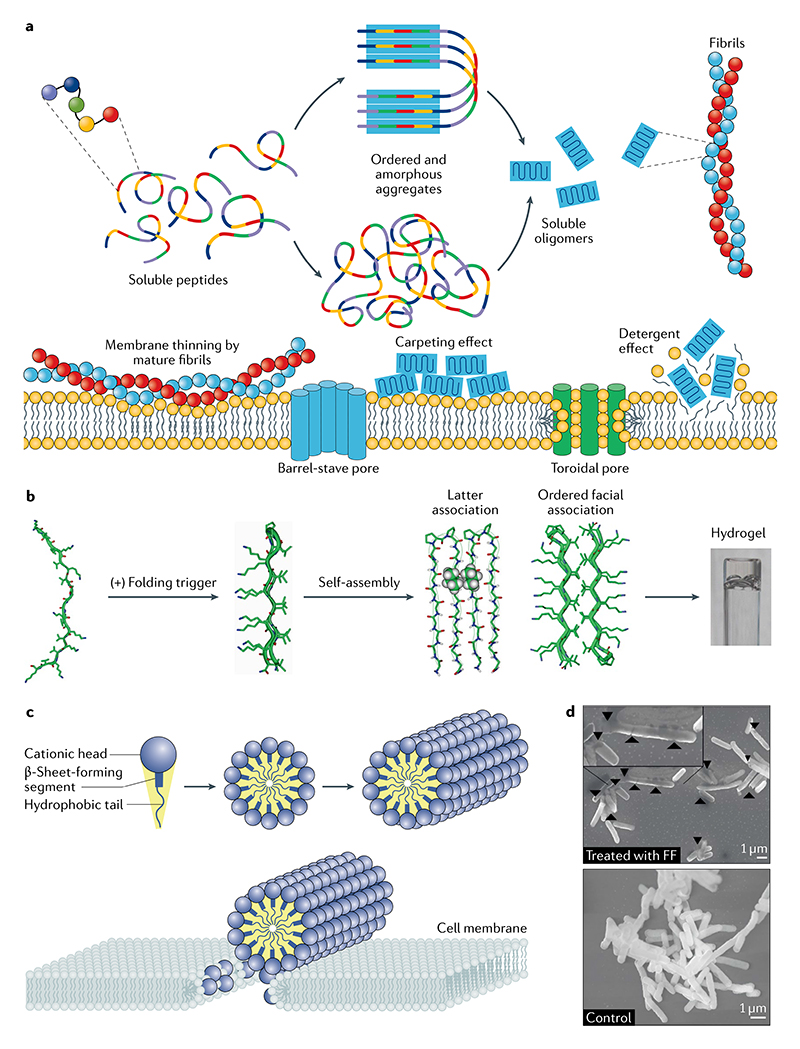
Fig. 4. Self-assembling biomimetic-peptide-based antimicrobial nanostructures.
a | Different peptide-membrane interactions are proposed to give rise to antibacterial functions. b | The MAX1 peptide undergoes environmentally triggered folding, selfassembly and non-covalent fibril-crosslinking processes to give a hydrogel114. c | The supramolecular nanofibres formed by self-assembling peptide amphiphiles present cationic peptide sequences that are essential to their proposed mode of action140. d | Scanning electron micrographs of Escherichia coli with and without diphenylalanine. This dipeptide forms nanostructures that have clear effects on bacterial morphology141. FF, diphenylalanine. Part b adapted from REF.113, Springer Nature Limited. Part c adapted with permission from REF.140, American Chemical Society. Part d adapted from REF.141, CC BY 4.0.