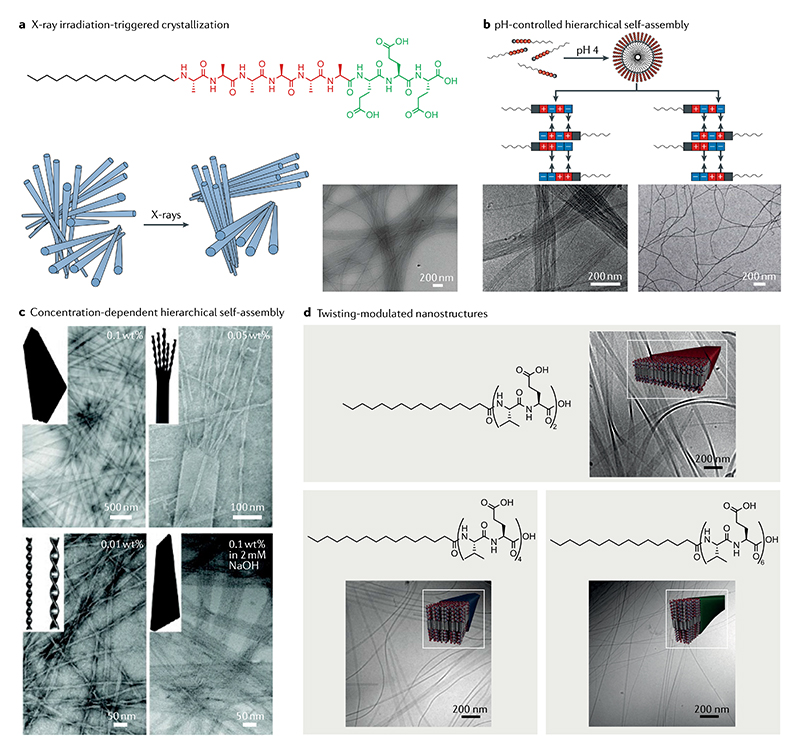
Fig. 5. Hierarchical self-assembly and crystallization of amphiphilic peptides.
a | X-ray-triggered phase transition of amphiphilic peptide filaments from a disordered phase to a hexagonally ordered phase as shown in the schematic (left). The formation of filament bundles is confirmed by cryo-transmission electron microscopy (right)11. b | pH-controlled hierarchical self-assembly of amphiphilic peptide (top left) ranging from micelles (top right) to nanofibre bundles (middle-left and bottom-left) to nanofibres (middle-right and bottom-right). The nanofibre bundles are the result of the complementary-attractive interactions between the surface regions of interdigitating peptide amphiphiles, and well-dispersed nanofibres are the result of the complementary repulsive interactions84. c | Concentration-dependent and pH-dependent morphology transformation of a flat amphiphilic peptide nanobelt into twisted nanoribbons. Transmission electron microscopy images clearly display this structural evolution from a flat nanobelt to a split nanobelt with a broom morphology. Decreasing the monomer concentration leads to the formation of twisted nanoribbons as well as the transformation from a flat nanobelt to a grooved nanobelt with parallel nanochannels. These images reveal that the flat nanobelts formed in a hierarchically oriented organization manner85. d | Hierarchical self-assembly of amphiphilic peptides containing variable numbers of valine-glutamic acid dimeric repeats92. Part a is adapted with permission from REF.11, AAAS. Part b is adapted with permission from REF.84, ACS. Part c is adapted with permission from REF.85, ACS. Part d is adapted with permission from REF.92, ACS.