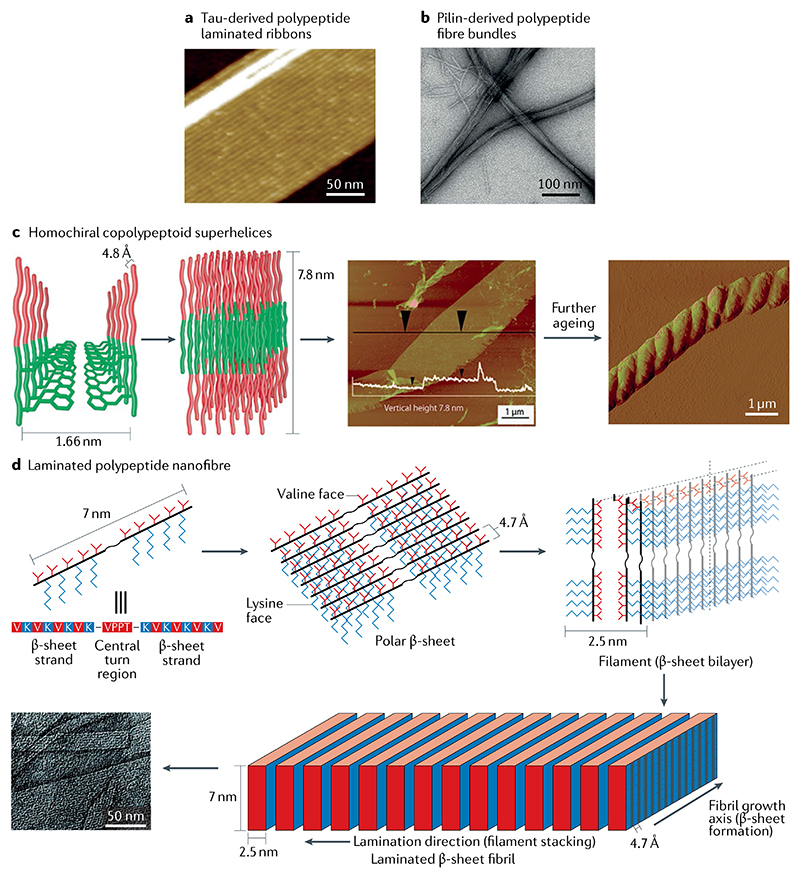
Fig. 6. Hierarchically oriented polypeptide self-assembly.
a | Multistranded ribbons self-assembled from a tau-derived polypeptide103. b | Fibre bundles self-assembled from a pilin-based polypeptide105. c | Helix formed from the lamellar stacked nanosheets of a diblock copolypeptoid. In the proposed hierarchical self-assembly model (left), the hydrophobic and hydrophilic portions of the chain are represented by green and red, respectively. Atomic force microscopy (AFM) image of the obtained layered structure (centre); the white overlay indicates the vertical height of the structure. AFM image of the helical structure that forms after further ageing (right)107. d | Flat fibril laminates formed through lateral association of β-sheet filaments. Polar β-sheets (top middle) with different hydrophobic (valine) and hydrophilic (lysine) faces are first formed from the self-assembly of polypeptide (top left) exhibiting an extended β-strand conformation. Subsequently, filaments (top right) are formed through the hydrophobic collapse of β-sheets. Lateral stacking of these filaments results in the formation of flat fibrils (bottom)109. Part a is reproduced with permission from REF.103, Wiley-VCH. Part b is adapted from REF.105, Springer Nature Limited. Part c is adapted with permission from REF.107, ACS. Part d is adapted with permission from REF109, ACS.