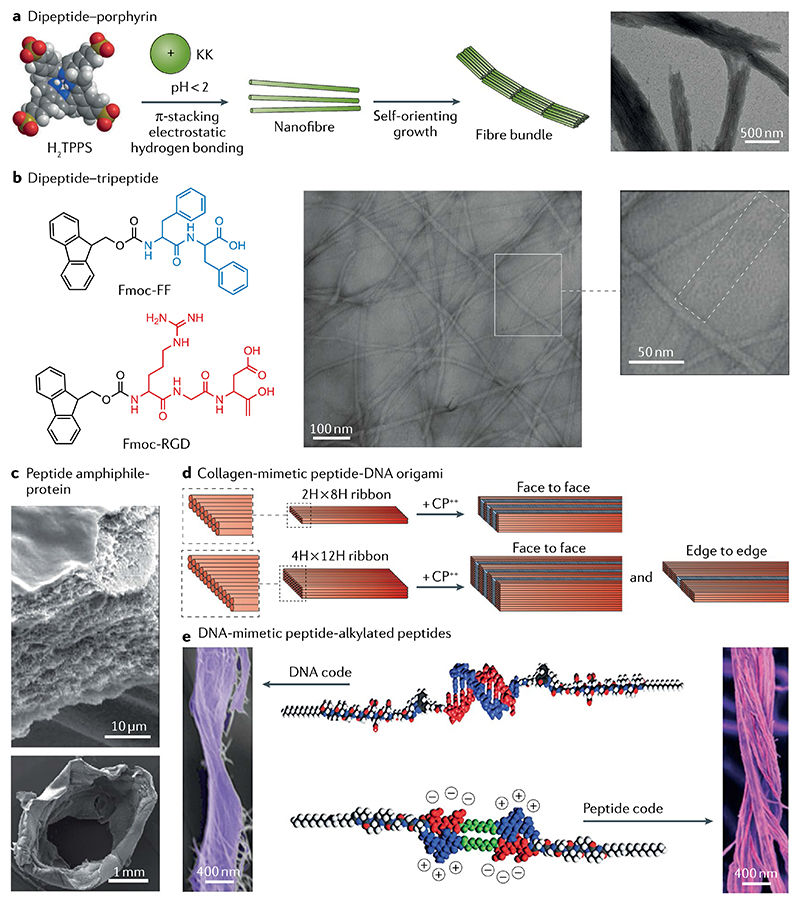
Fig. 7. Hierarchically oriented organization in peptide-containing co-assembly systems.
a | Long-range ordered nanofibre bundles assembled from a dipeptide (KK)–porphyrin co-assembly system due to the balance between electrostatic repulsion and van der Waals attraction113. b | Flat ribbons of 9-fluorenylmethyloxycarbonyl (Fmoc)–diphenylalanine (FF) and Fmoc–RGD co-assemblies comprising laterally aligned fine fibrils about 3 nm in diameter, as observed by fast Fourier transform analysis114. c | A protein–peptide co-assembly membrane containing elastin-like polypeptides and an amphiphilic peptide demonstrates pronounced nanofibrous multilayered architecture, as shown by scanning electron microscopy images of a cross-section of the membrane (top). The membrane can further evolve into tubular structures with simultaneously spatiotemporal control (bottom)116. d | Co-assembly of collagen-mimetic peptides (CP++) and DNA origami yields the formation of nanowires with repeating periodicity of about 10 nm through face-to-face or edge-to-edge packing modes118. e | Bundles of intertwined fibres formed through co-assembly of DNA-mimetic peptide amphiphiles (top) and alkylated peptides (bottom)125. Part a is adapted with permission from REF.113, Wiley-VCH. Part b is adapted with permission from REF.114, Elsevier. Part c is reproduced from REF.116, Springer Nature Limited. Part d is reproduced with permission from REF.118, ACS. Part e is reproduced with permission from REF.125, AAAS.