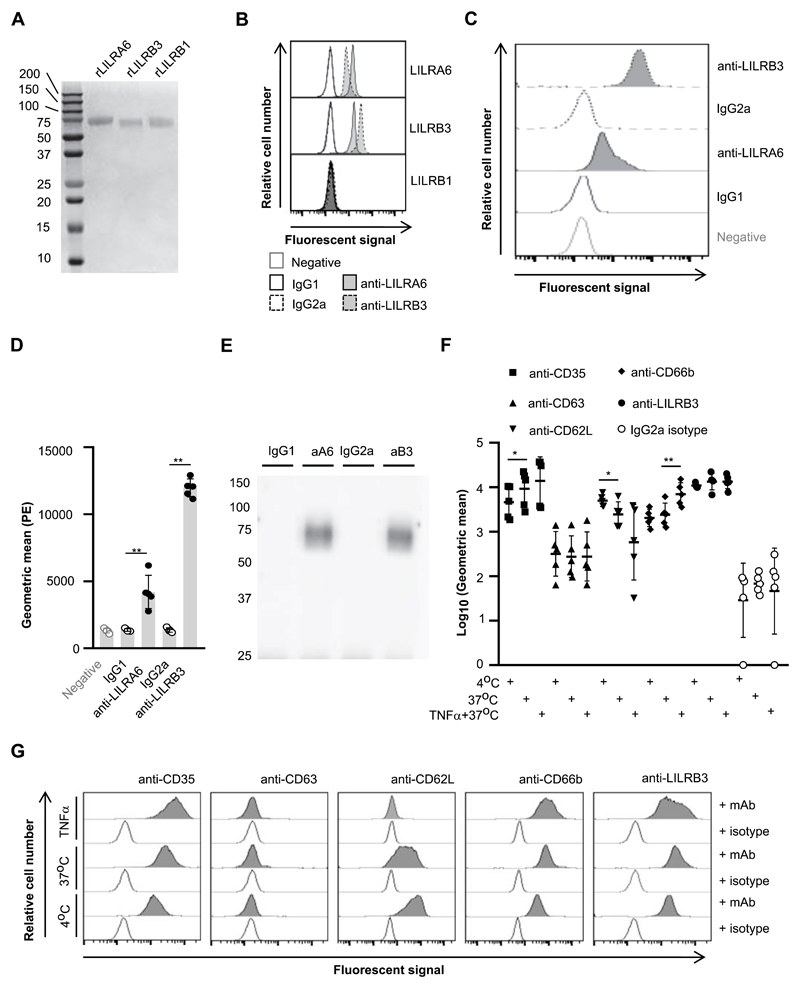
Figure 1. LILRB3 is expressed on the surface of resting neutrophils, and is down-regulated upon priming.
(A) Seperation of recombinant (r)LILRA6, LILRB3 and LILRB1 purified from eukaryotic expression system by SDS-PAGE. (B) Binding of anti-LILRA6, anti-LILRB3 and isotype control mAb to Dynabeads coated with rLILR. Representative flow cytomerty plots of n = 3 are shown. (C and D) Binding of anti-LILRA6, anti-LILRB3 and isotype control mAb to resting neutophils, using anti-IgG-PE as a seconday mAb. A representative experiment (C) and the integrated results from five separate experiments (D) were compared by Student t-test, where * = p < 0.05, ** = p < 0.01. (E) Immunoprecipitation of LILRA6 and/or LILRB3 from the surface of resting neutrophils. Neutrophil lysates were incubated with 5 μg/ml anti-LILRA6 (aA6), anti-LILRB3 (aB3), IgG1 or IgG2a (i) and DB protein G. Immunoprecipitated proteins were eluted from beads, seperated via SDS-PAGE, blotted onto membranes and detected using rabbit anti-LILRB3 pAb or rabbit IgG and goat anti-rabbit-IgG-HRP. Data is representative of n = 3 independent experiments. (F and G) Priming of neutrophils with TNFα does not alter LILRA6/B3 expression levels. Neutrophils were incubated at 4°C (resting), at 37°C (secretory vesicles exocytosed) or 37°C in the presence 5 μg/ml TNFα (primary granules exocytosed). Anti-IgG-PE used as a secondary mAb for anti-LILRB3 and IgG2a isotype control. Integrated results from five separate experiments (F) were compared by Student t-test, where * = p < 0.05, ** = p < 0.01. Data from a representative experiment are shown (G).