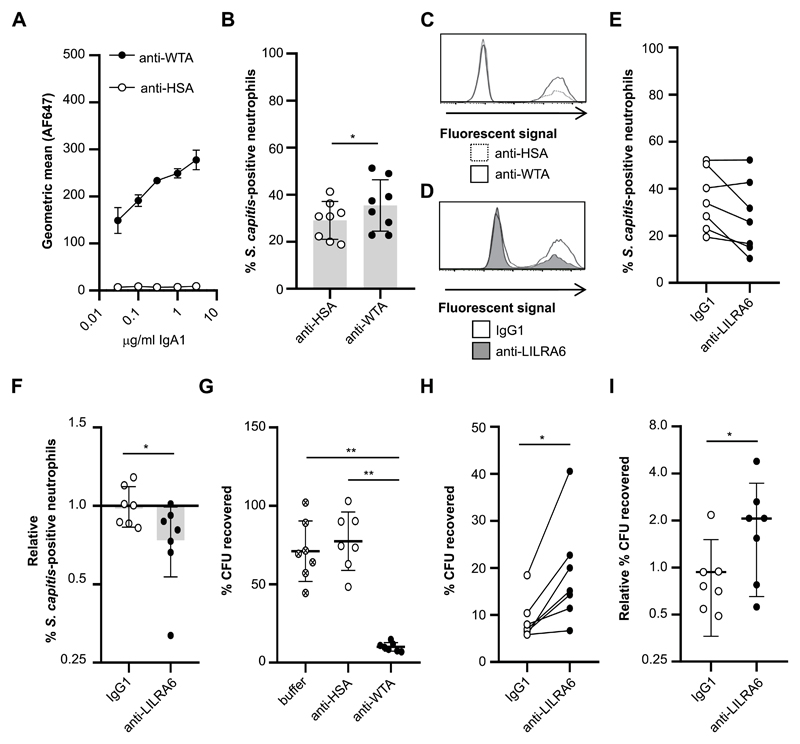
Figure 6. LILRB3 suppresses FcαR-mediated antimicrobial effector functions.
(A) Concentration-dependent binding of anti-WTA-IgA1 but not an isotype control to Staphylococcus capitis ATCC 27840-H strain. n = 3. (B and C) Phagocytic uptake of IgA-opsonised S. capitis by neutrophils at an MOI of 10:1. Neutrophils were incubated in the presence of 5 μg/ml FLIPr-like for 20 minutes, and then in the presence of anti-WTA-IgA1-opsonised FITC-labelled S. capitis for 30 minutes at 37°C with 5% CO2. Fluorescence of neutrophils was measured by flow cytometry analysis. The % of fluorescent neutrophils was calculated for each neutrophil population. The integrated results from eight separate donors (B) were compared by Student t-test, where * = p < 0.05, ** = p < 0.01, and representative flow cytometry plots are shown (C). (D, E and F) Continuous ligation of LILRB3 reduced phagocytic uptake of S. capitis at an MOI of 10:1. Neutrophils were incubated in the presence of 5 μg/ml FLIPr-like for 20 minutes, and then incubated on anti-LILRA6 or IgG1 coated plates. After 1 hour, neutrophils were incubated in the presence of anti-WTA-IgA1-opsonised FITC-labelled S. capitis for 30 minutes at 37°C with 5% CO2. Fluorescence of neutrophils was measured by flow cytometry analysis. The % of fluorescent neutrophils was calculated for each neutrophil population. A representative experiment (D) and the integrated results from seven separate donors (E), in which data from the same donor are linked, are shown. Relative % of fluorescent neutrophils (F) was calculated by normalizing values of against neutrophils pre-incubated on buffer control coated wells, and was compared by Student t-test, where * = p < 0.05, ** = p < 0.01. (G) Killing of IgA-opsonised S. capitis by neutrophils at a MOI of 1:1. Neutrophils were incubated in the presence of S. capitis opsonised with anti-WTA-IgA1 or anti-HSA-IgA or buffer control, for 60 minutes at 37°C with 5% CO2. Following neutrophil lysis, the % of CFU recovered at 60 minutes compared to 0 minutes was quantified by serial dilution and growth on BHI agar plates. Data from one donor was removed as an outlier using ROUT method. Data was analysed by Students t test, where * = p < 0.05, ** = p < 0.01. n = 7. (H and I) Continuous ligation of LILRB3 inhibited bacterial killing. Neutrophils were incubated on anti-LILRA6 or IgG1 coated plates for 1 hour prior to incubation for 60 minutes at 37°C with 5% CO2 in the presence of S. capitis opsonised with anti-WTA-IgA1 or anti-HSA-IgA or buffer control. After neutrophil lysis, the % of CFU recovered at 60 minutes compared to 0 minutes was quantified by serial dilution and growth on BHI agar plates. The integrated results from seven separate donors (H), in which data from the same donor are linked, were compared by Wilcoxon matched-pairs signed rank test, where * = p < 0.05, ** = p < 0.01. Relative % of recovered CFU (I) was calculated by normalizing values of neutrophils pre-incubated on anti-LILRA6 or IgG1 coated wells to buffer control, and compared Wilcoxon matched-pairs signed rank test, where * = p < 0.05, ** = p < 0.01.