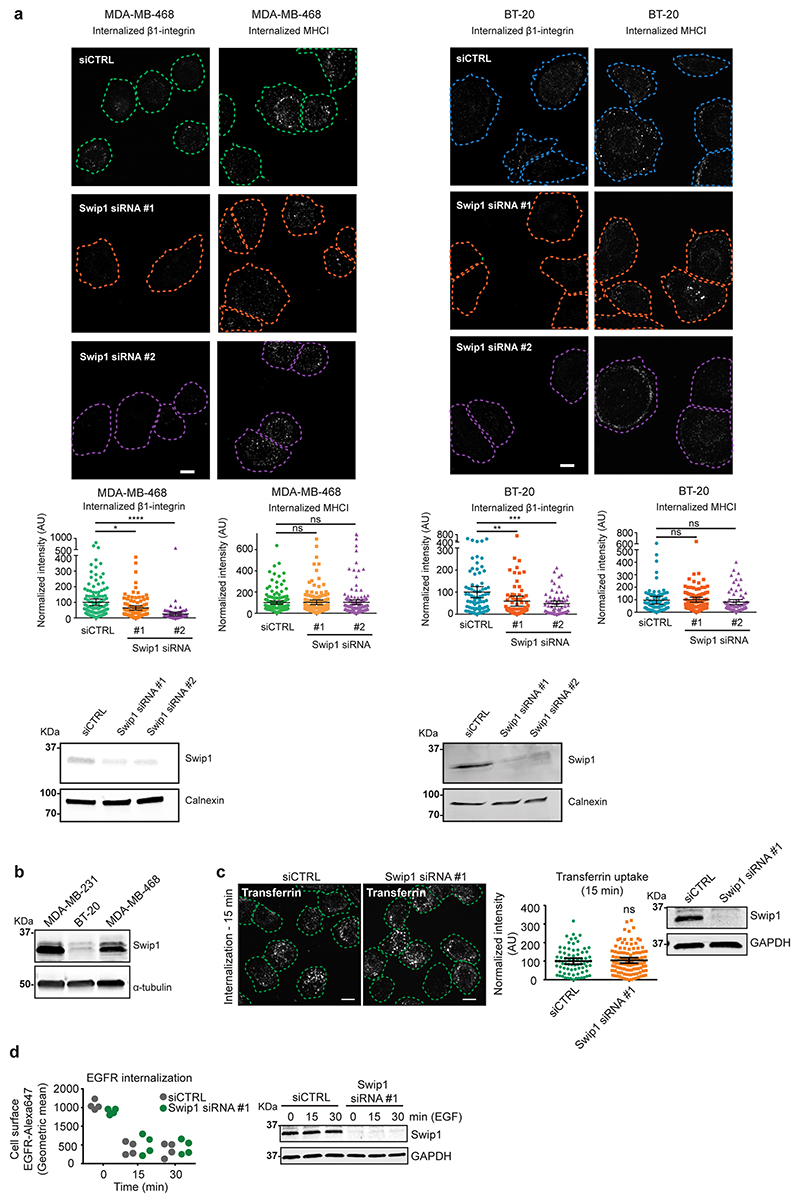
Extended Data fig. 6. Swip1 regulates β1-integrin endocytosis via the CG pathway.
(a) Representative micrographs and quantification of β1-integrin and MHCI uptake at the 15 min time point in control or Swip1 (siRNA#1 or #2) silenced MDA-MB-468 and BT-20 cells. Representative immunoblots of cell lysates blotted for Swip1. Calnexin is included as a loading control. Scale bars, 10 μm. (b) Representative immunoblot of MDA-MB-231, BT-20 and MDA-MB-468 cell lysates blotted for Swip1. α-tubulin is included as a loading control. (c) Representative micrographs and quantification of transferrin uptake at the 15 min time point in control- or Swip1-silenced MDA-MB-231 cells. Representative immunoblots of cell lysates blotted as indicated. Calnexin is included as a loading control. Scale bars, 10 μm. (d) Cell-surface labelled EGFR uptake in control and Swip1-silenced MDA-MB-231 cells treated with 10 ng/ml EGF was analysed using flow cytometry in 10 000 non-permeabilized cells per measurement. Plot shows 4 measurements from n = 2 independent experiments. Immunoblot to validate swip1 silencing is representative of 2 independent experiments. GAPDH is included as a loading control. Immunoblots in panels (a-c) and scatter dot-plots (a-c) are representative of 3 independent experiments. Data are presented as mean values ± 95% CI. Statistical significance was assessed with two-sided Mann–Whitney tests, where n is the total number of cells pooled across 3 independent experiments. P values calculated compared to siCTRL condition: *P = 0.0137, **P = 0.0007, ***P = 0.0006, ****P<0.0001, ns = not significant. Number of analysed cells over 3 independent experiments: (a) For BT-20 12G10 and MHCI uptake, respectively, siCTRL, n = 79 & 91 cells; Swip1 siRNA#1, n = 76 & 91 cells; Swip1 siRNA#2, n = 66 & 75 cells. For MDA-MB-468 12G10 and MHCI uptake, respectively, siCTRL, n = 134 & 132 cells; Swip1 siRNA#1, n = 98 & 117 cells; Swip1 siRNA#2, n = 98 & 101 cells. (c) siCTRL, n = 74 cells; Swip1 siRNA#1, n = 103 cells. Unprocessed blots and numerical source data are provided in Source data.