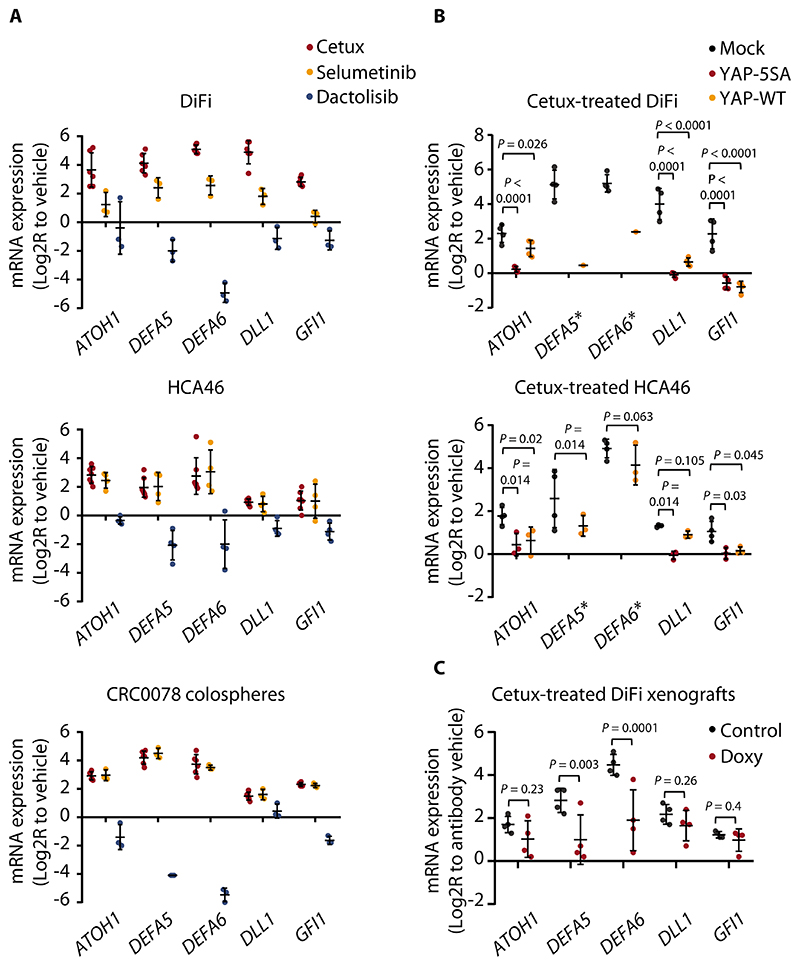
Figure 4. Acquisition of the Paneth cell-like phenotype in residual cells involves YAP inactivation.
(A) RT-qPCR analysis of the indicated secretory/Paneth-cell markers in DiFi and HCA46 cells and in CRC0078 colospheres treated with cetuximab (20 μg/ml), selumetinib (1 μM), or dactolisib (250 nM) for 72h. Independent experiments (n = 3 for DiFi and CRC0078; n = 4 for HCA46) were performed in technical triplicates. In some experiments, controls (vehicle) with untreated cells were repeated. The plots show means ± SD. The effects of cetuximab and selumetinib were positively correlated (DiFi, r = 0.857; HCA46, r = 0.963; CRC0078, r = 0.984, Pearson’s correlation coefficient), whereas dactolisib-induced transcriptional changes tended to negatively correlate with those triggered by cetuximab (DiFi, r = -0.592; HCA46, r = -0.104; CRC0078, r = -0.905). Cetux, cetuximab. (B) RT-qPCR analysis of the indicated secretory/Paneth-cell genes in DiFi and HCA46 cell lines transduced with a pLVX-control vector (mock), the constitutively active YAP variant (YAP-5SA), or wild-type YAP (YAP-WT) and treated with cetuximab (20 μg/ml) for 72h. Independent experiments (n = 3 for HCA46 and n = 4 for DiFi) were performed in technical triplicates. In some experiments, controls (vehicle) with untreated cells were repeated. The plots show means ± SD. Statistical analysis by one-way ANOVA followed by Benjamini, Krieger and Yekutieli FDR correction. Stars next to DEFA5 and DEFA6 indicate loss ofbasal expression in the YAP-5SA experimental group, which prevented statistical comparison against mock controls for these genes. In the DiFi YAP-WT group, DEFA5 and DEFA6 expression was above detection in one replicate. (C) RT-qPCR analysis of the indicated secretory/Paneth-cell genes in established DiFi xenografts transduced with doxycycline-inducible YAP-5SA and treated with vehicle (control), cetuximab (20 mg/kg, intraperitoneal injection, 1 administration), doxycycline (Doxy, 50 mg/kg, daily oral gavage), or the combination of cetuximab and doxycycline for 1 week. Results from animals treated with cetuximab were normalized against those from animals treated with vehicle. Results from animals treated with cetuximab and doxycycline were normalized against those from animals treated with doxycycline. Four samples for each condition were analyzed in technical triplicates. The plots show means ± SD. Statistical analysis by one-way ANOVA followed by Benjamini, Krieger and Yekutieli FDR correction.