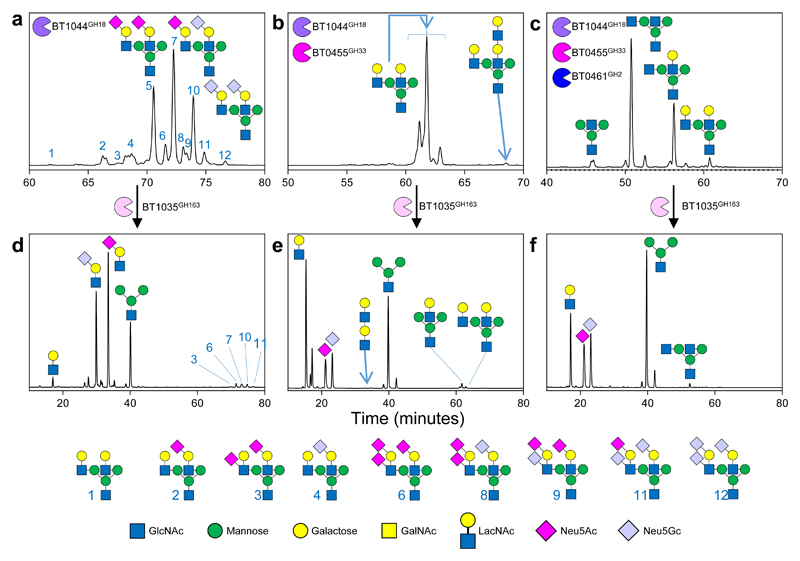
Figure 3. The degradation of biantennary CNG by recombinant enzymes from Bt.
a, α1AGp digested with BT1044GH18 endo-GlcNAc’ase only and b, then in combination with BT0455GH33 sialidase, and c, then also BT0461GH2 β-galactosidase. d-f, Each of the assays shown in a-c were stopped after a 24 h incubation using heat denaturation and BT1035GH163 then added. The time shown for the different chromatograms varies between panels to provide clarity of the main peaks. The glycan products were labelled with procainamide and analysed by LC-FLD-ESI-MS (see Materials and Methods). The most abundant glycan products are annotated on the chromatograms and the minor products are shown in the key at the bottom. The same glycan species detected in multiple peaks is likely due to different linkages and glycosylation on different arms, which cannot be determined by the analytical methods employed. Neu5Ac and Neu5Gc are pink and light blue, respectively. The linkages they are attached through could not be determined using the techniques employed here and also likely vary between glycans and glycoproteins. The results are representative of at least three independent replicates. A sugar key is included in Figure 1.