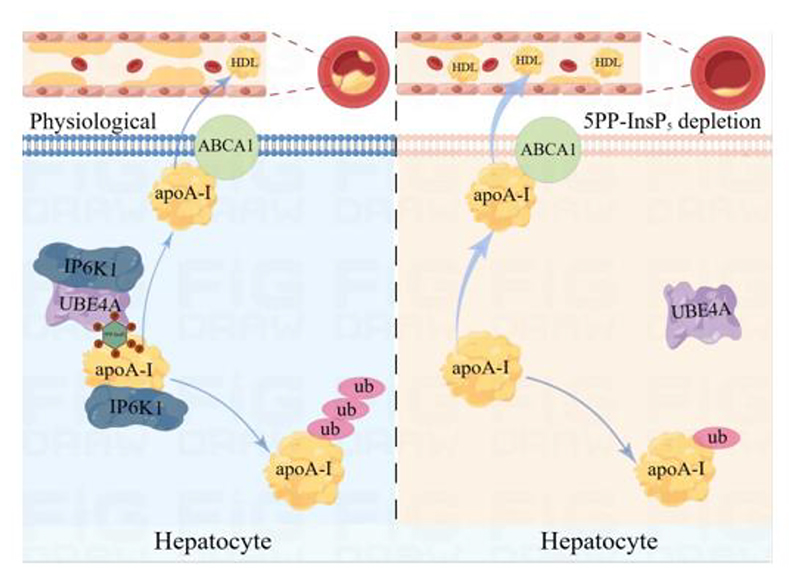
Fig. 7. Model of 5PP-InsP5 depletion increasing apoA-I expression, augmenting reverse cholesterol transport, and attenuating atherosclerosis.
(Left) IP6K1 physiologically binds to apoA-I and UBE4A. A local pool of 5PP-InsP5 was produced by IP6K1 to enhance the interaction of apoA-I with UBE4A. This leads to apoA-I ubiquitination and degradation. (Right) Depleting 5PP-InsP5 by genetic deletion or pharmacological inhibition of IP6K1 disrupts the binding of UBE4A to apoA-I. This allows apoA-I to escape from degradation. ABCA1 interacts with apoA-I to facilitate the formation of nascent HDL, which is then released into the plasma. The higher levels of apoA-I are linked to enhanced reverse cholesterol transport activity, which reduces atherosclerosis.