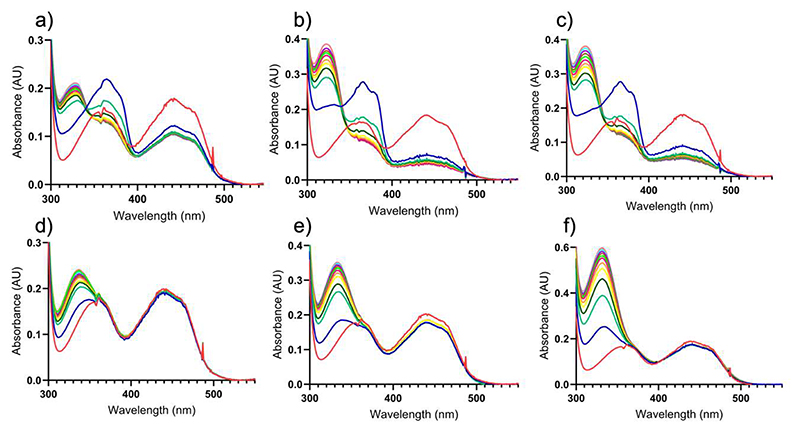
Figure 4. Spectral changes observed upon aerobic mixing VAD with 4-(methoxy)methylphenol.
(a-c) 17 μM wild-type VAD mixed with (a) 17 μM, (b) 34 μM, and (c) 68 μM 4-(methoxymethyl)phenol. (d-f) 17 μM P151L VAD mixed with (d) 17 μM, (e) 34 μM, (f) 68 μM 4-(methoxymethyl)phenol. The spectra represent the enzymes before (red) and immediately after the addition of 4-(methoxymethyl)phenol (blue) followed by incubations for 30 seconds (green), 1 minute (dark green), 1.50 minute (yellow), 2 minutes (orange), 2.50 minutes (purple), 3 minutes (light green), 3.50 minutes (brown), 4 minutes (violet), 4.50 minutes (light blue) and 5 minutes (pink). The quinone methide intermediate initially formed upon 4-(methoxy)methylphenol oxidation absorbs at 364 nm (ε=46000 M−1·cm−1) and is clearly visible in the early incubation times of the wild-type protein. The intermediate is then attacked by a water to generate 4-hydroxybenzaldehyde that has an absorbance peak at 329 nm (ε=10600 M−1·cm−1; Scheme 1d).