Figure 3.
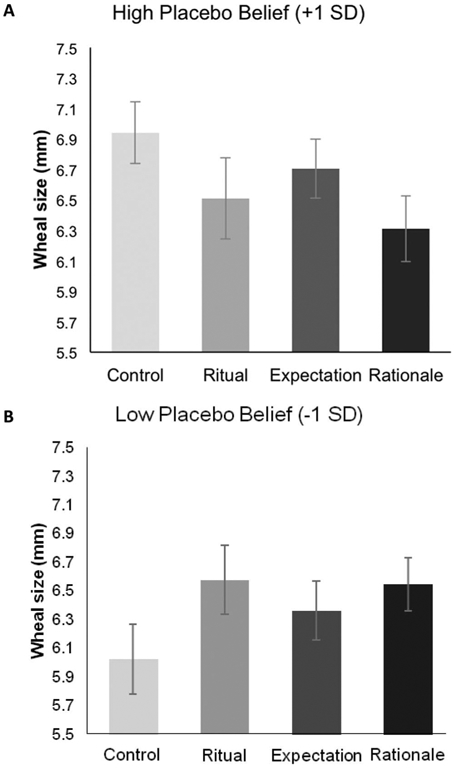
Interaction between participants’ allergic reactions and their belief in placebos. Participants’ allergic reactions were measured via wheal size by a research assistant blind to participant condition. Panels (A) and (B) display adjusted means at T2 (immediately after interacting with the provider at 9 min post-skin prick test) derived from a multilevel longitudinal model controlling for wheal size at T1 (3 min post-skin prick test). Panel (A) shows participants high on the belief in placebos scale (+1 SD), and panel (B) shows participants low on the belief in placebos scale (−1 SD). SEs represent ±1 SE. * p < .05.
