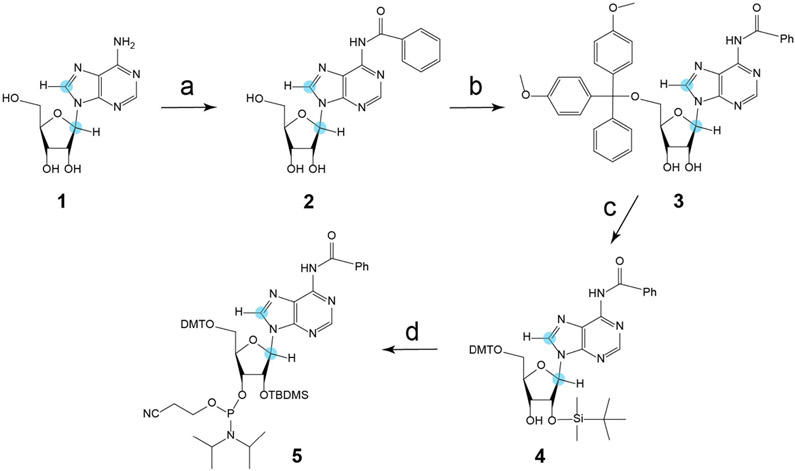
Scheme 1.
Synthesis of N6-benzoyl-8,1′-13C-2′-O-TBDMS-adenosine phosphoramidite 5. Reaction conditions: a) Benzoylchloride, trimethylsilyl chloride, pyridine, 2.5 h, rt, then water, 28% NH3, 39%. b) Di-tert-butylsilyl bis(trifluoromethanesulfonate) in DMF, 0 °C, 1 h, then tert-butyldimethylsilyl chloride, 3 h, 60 °C, 40%. c) HF•Py in CH2Cl2, 0 °C, 1 h, then DMT-Cl in pyridine, rt, 3 h, 91%. d) CEP-Cl, DiPEA in THF, rt, 3 h, 80%. Orange dot = 13C.