Figure 6. Caspase Limits Diverse p38 MAPK-Dependent Stress Responses.
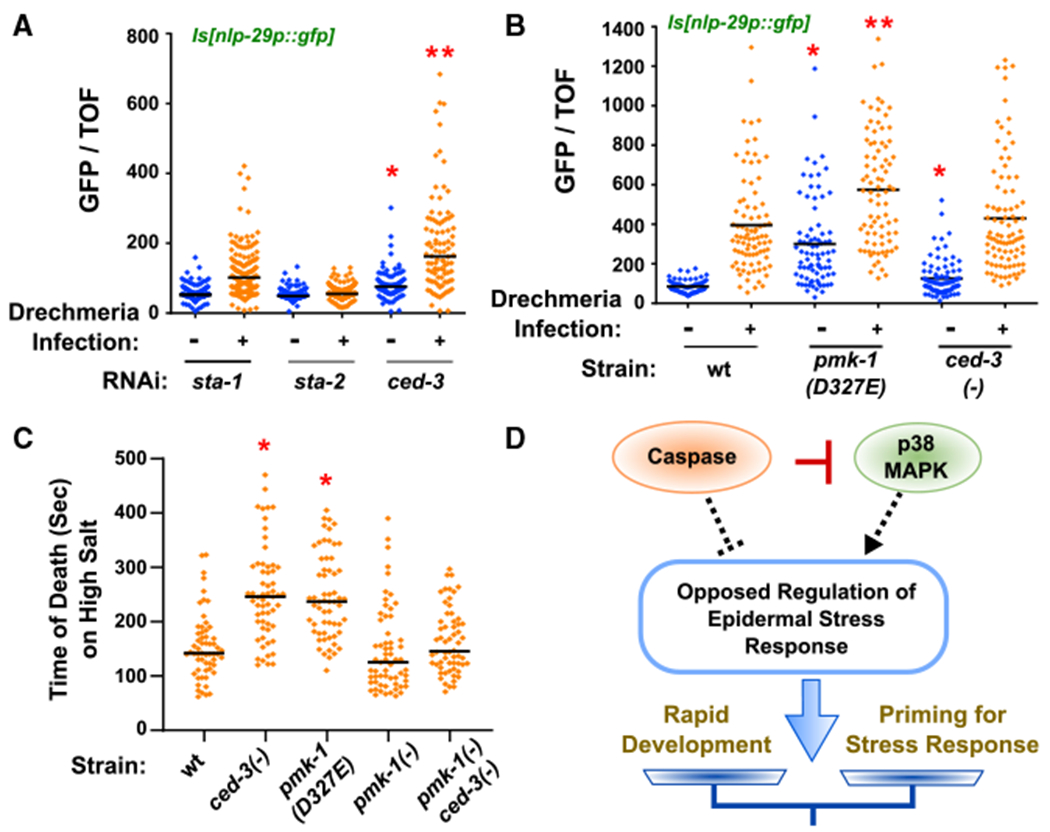
(A and B) Loss of ced-3 by RNAi and pmk-1(D327E) primes infected animals for expression of anti-microbial nlp-29p::gfp. Expression of nlp-29p::gfp in animals with and without Drechmeria infection and indicated RNAi treatments (A) or mutant strains (B). Vertical axis shows the intensity of green animals normalized by size (time of flight, TOF). (A) *Significant, ced-3(RNAi) versussta-1(RNAi) before infection, p < 0.0001, Mann-Whitney. *Significant, ced-3(RNAi) versus sta-1(RNAi) after infection, p < 0.0001, Mann-Whitney. Median values (thin horizontal bars). (B) *Significant, pmk-1(D327E) versus wt before infection, p < 0.0001, and ced-3(−) versus wt before infection, p = 0.0068, Mann-Whitney. **Significant, pmk-1(D327E) after infection, p < 0.0001, Mann-Whitney.
(C) Loss of ced-3 or loss of CED-3 cleavage site in pmk-1 primes animals for acute hyperosmolarity stress resistance. *Significant, wt versus ced-3(−) and pmk-1(D327E), p < 0.001, Mann-Whitney, median values (bars).
(D) Diagram of opposed regulation of ~300 genes by caspase and p38 to balance stress responses and rapid development. CED-3 directly blocks the action of p38 by proteolytic cleavage (red bar). By inversely regulating the epidermal stress response (dashed lines), ced-3 and pmk-1 fine tune the extent of stress priming. The net outcome (arrow) based on inputs alters the balance of stress response and developmental progression (scale).
