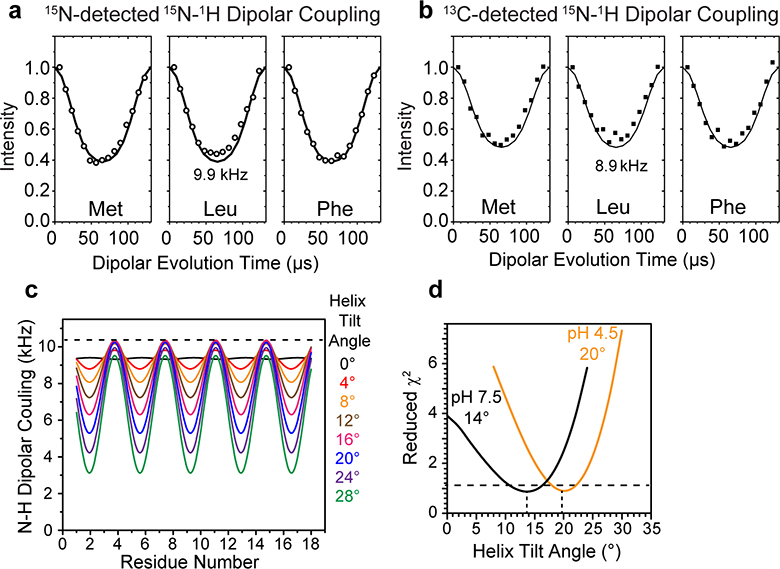
Extended Data Fig. 6. Measurement of BM2 helix orientation using rotationally averaged 15N-1H dipolar couplings.
(a-b) N-H DIPSHIFT data of the tripeptide formyl-MLF, measured at Tsample = 315 K using (a) 15N detection and (b) 13C detection. The dipolar-doubled version of DIPSHIFT is used in these experiments. The 15N-detected DIPSHIFT data were analyzed using the total intensities from the centerband and sidebands. The 13C-detected N-H couplings used a 15N-13C TEDOR mixing time of 2.11 ms. The 13C-detected N-H couplings are 0.9 times the 15N-detected values, indicating incomplete powder averaging. This scaling factor was included in determining the BM2 orientation from 13C-detected N-H dipolar couplings. (c) Calculated 15N-1H dipolar waves as a function of the helix tilt angle. An 18-residue ideal α-helix with (ϕ, ψ) angles of (−65˚, −40˚) were tilted from an external axis by 0°–30°. The 15N-1H dipolar couplings show the expected sinusoidal oscillations with a periodicity of 3.6 residues. The amplitude and offset of the dipolar wave indicate the helix tilt angle. (d) Reduced χ2 values of the measured and simulated 15N-1H dipolar couplings of membrane-bound BM2 at high and low pH. The minimum χ2 value is found at a tilt angle of 14˚ for high-pH BM2 and 20˚ for low-pH BM2. The ±2˚ uncertainty represents one standard deviation.