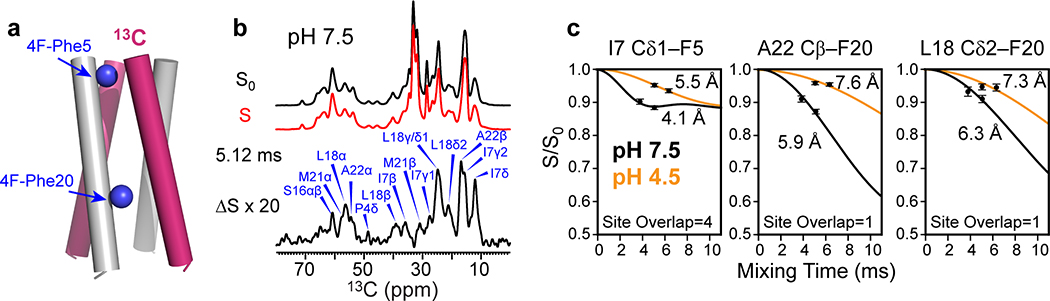
Figure 3.
Determination of interhelical distances of membrane-bound BM2 using 13C-19F REDOR. (a) Schematic of mixed fluorinated and 13C-labeled protein for measuring interhelical distances. (b) Representative 1D 13C-19F REDOR control (S0) and dephased (S) spectra, whose difference (ΔS) shows the signals of 13C spins that are in close proximity to the fluorinated Phe5 and Phe20. The 13C-19F REDOR spectra were measured in gel-phase membranes at a Tsample of 273 K for the POPE-bound BM2 at high pH and 261 K for the POPC : POPG-bound protein at low pH. (c) Representative 13C-19F REDOR dephasing curves. Weaker dephasing is observed for the low-pH protein, indicating longer interhelical distances. The site overlap factor for each site is indicated.