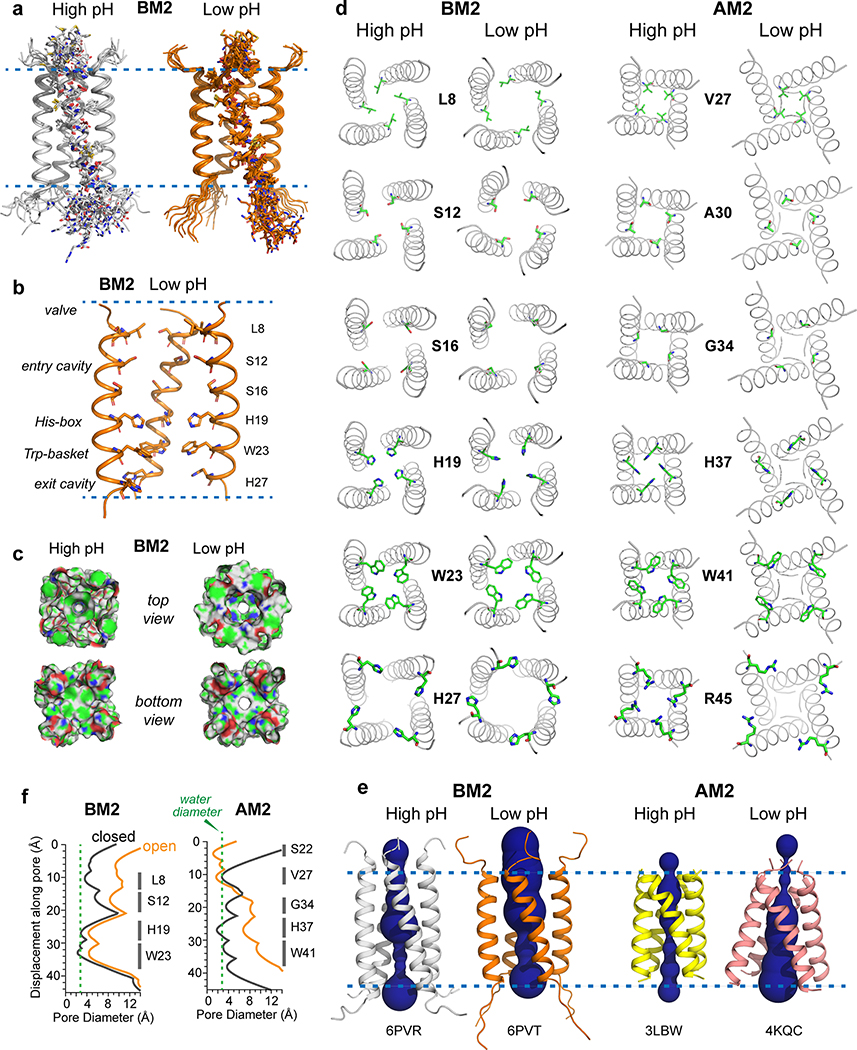
Figure 4.
Atomic structures of closed and open BM2 channels in lipid membranes. (a) Ensembles of ten lowest-energy structures, showing the backbones of all four helices and sidechains of one of the four helices. (b) Lowest-energy structure of the open channel, showing key sidechains along the pore. One of the four helices is omitted for clarity. (c) Solvent-excluded surface representations of closed (high pH) and open (low pH) BM2 and AM2 channels, viewed from the N-terminus (top view) and C-terminus (bottom view). Atoms coloring is green for carbons, white for hydrogens, red for oxygens, and blue for nitrogens. (d) C-terminal views of the channel pores and sidechain structures of six key residues in the closed and open BM2 and AM2. The open BM2 channel shows a larger N-terminal pore and tighter Trp gate compared to the open AM2. (e) Side view of the closed and open BM2 and AM2 channels, showing HOLE-calculated water-permeated pores. The average pore diameter for BM2 is 6.6 Å at high pH and 8.7 Å at low pH. (f) Pore diameters of BM2 and AM2 calculated using the HOLE program. The diameter of water molecules is shown as a green dashed line. Activation of BM2 enlarges the pore diameter along the entire channel axis, corresponding to a scissor motion, whereas activation of AM2 constricts the N-terminus while splaying the C-terminus, corresponding to a hinge motion.