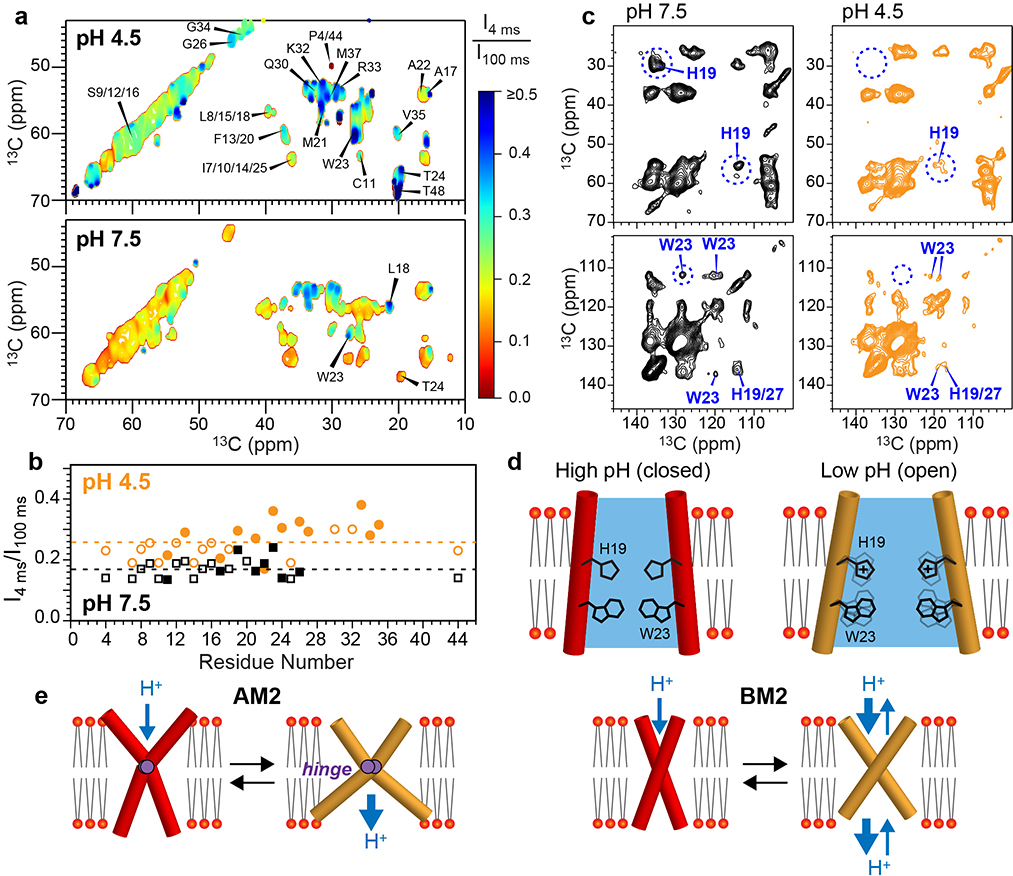
Figure 5.
Water accessibilities and sidechain motions of BM2 in the high-pH (closed) and low-pH (open) states. (a) Normalized water-edited 2D 13C-13C correlation spectra of BM2 at low and high pH, measured at Tsample = 273 K. The low-pH channel shows higher water-transferred intensities (blue contours) than the high-pH channel (red contours). (b) Intensity ratios of water-edited and full spectra. The low-pH channel shows higher intensities than the high-pH channel, and the C-terminus is more hydrated than the N-terminus. The intensities of resolved and overlapped signals are shown as closed and open symbols, respectively. Dashed lines indicate the average S/S0 values for the two pH conditions. (c) 2D 13C-13C correlation spectra of membrane-bound BM2 at Tsample = 280 K. H19 and W23 peaks are much weaker at low pH than at high pH, indicating that these residues are mobile in the open state. (d) Schematic of the larger water accessibility and increased HxxxW sidechain motions of BM2 at low pH. (e) Schematic models of AM2 and BM2’s backbone motion. AM2 undergoes an alternating-access hinge motion to conduct protons only inward, whereas BM2 undergoes a symmetric scissor motion to conduct protons both inward and outward.