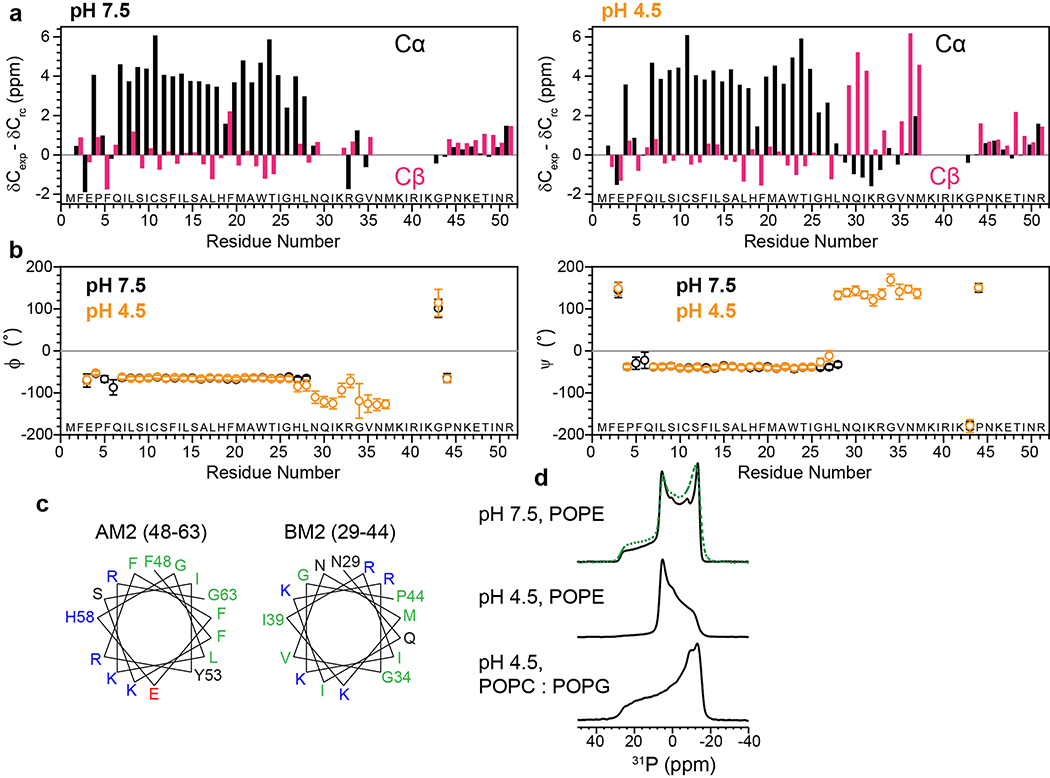
Extended Data Fig. 4. Secondary structure of BM2 in the closed (high pH) and open (low pH) states.
(a) Cα (black) and Cβ (magenta) secondary chemical shifts at pH 7.5 and pH 4.5. (b) Chemical-shift derived (ϕ, ψ) torsion angles at pH 7.5 (black) and pH 4.5 (orange). At both pH, the TM domain is α-helical while the cytoplasmic tail is mostly disordered. In addition, a short β-strand segment is present at low pH. (c) Helical wheel representations of residues 48–63 in AM2 and the corresponding residues 29–44 in BM2. Hydrophobic residues are colored green, polar residues black, positively charged residues blue, and negatively charged residues red. AM2 has a separate hydrophobic face and a hydrophilic face, indicative of an amphipathic helix, while BM2 has alternating polar and non-polar residues, consistent with a β-strand conformation. (d) Static 31P spectra of BM2-containing POPE membrane at high and low pH and POPC/POPG membranes at low pH, all measured at a sample temperature of 303 K. At high pH the POPE membrane consists of ~65% bilayer and ~35% hexagonal phase. At low pH BM2 converts most of the POPE membrane to the hexagonal phase, but retains the lamellar form for the POPC/POPG membrane. Green dashed line is a superposition of 35% of the pH 4.5 POPE spectrum and 65% of the pH 4.5 POPC : POPG spectrum.