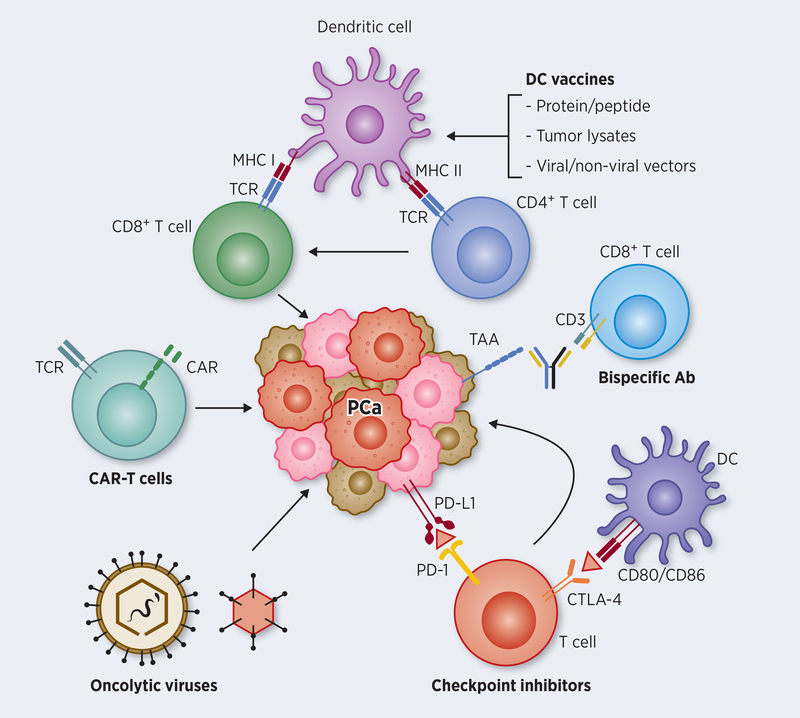
Figure 1: Major immunotherapy pathways targeting PCa cells.
Attempts to activate tumor-specific CD8+ T cells against prostate cancer involved loading dendritic cells (DCs) with proteins and peptides of tumor antigens or transducing antigen genes into DCs using viral and non-viral vectors by ex vivo or in vivo approaches. Such antigen-loaded DCs, prompted by additional signals for maturation and APC function results in augmenting CTL effector function, both in number and in activity. Optimizing DC function further enables CD4+ T cells promote T helper function against growing tumor. Genetic approaches to harness tumor-specific CD8+ T cells directly involves harvesting T cells from prostate cancer, transfecting them with chimeric antigen receptor (CAR) genes directed against the patients’ tumor, expanding the modified T cells ex vivo, and reinfusing them back into the patients for antitumor activity. Bispecific antibody conjugates help direct tumor-specific CD8+ T cells to tumor target using an adapter molecule involving CD3 and a tumor surface antigen-specific antibodies. Whereas the above approaches help in the generation and activation of tumor-specific T cells, immune checkpoint inhibitors (ICIs) on the other hand help in blocking inhibitory pathways that dampen T cell function. The two major checkpoint molecules on T cells that block effector function are PD1 and CTLA-4 that interact with PD-L1 produced by tumor cells, and CD80/86 on APCs, respectively. Recent immunotherapy approaches using monoclonal antibody blockade of their inhibitory interaction are highly promising in the clinic to improve CTL function. Oncolytic viruses, which are engineered to selectively replicate and kill tumor cells further improve immunotherapy approaches for effective cross-presentation of tumor antigens to the immune system, either as standalone treatment or in combination with other immunotherapy approaches.