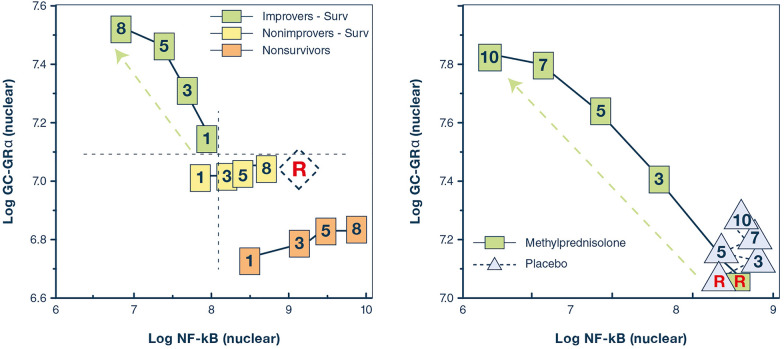
Fig. 1.
Relations on natural logarithmic scales between mean levels of nuclear NF-κB and nuclear GC-GRα during the natural progression of ARDS, and in response to prolonged methylprednisolone treatment. Mean intracellular changes of nuclear GC-activated GRα and NF-κB were observed by exposing peripheral blood mononuclear cells of a healthy volunteer to ARDS patient plasma samples collected longitudinally (days 1, 3, 5, and 8) and after randomization to methylprednisolone treatment (randomization day (R) and post-randomization days 3, 5, 7, and 10). The mean values of nuclear NF-κB are plotted against the mean nuclear GC-GRα levels. Improvers had a pre-defined improvement in lung injury score [45] and/or gas exchange component by day 7. The left panel shows ARDS patients with adaptive and maladaptive responses. In improvers, an inverse relationship was observed between these two transcription factors, with the longitudinal direction of the interaction shifting leftwards (decreased NF-κB) and upward (increased GC-GRα). Conversely, in non-improvers, NF-κB increased over time, while GC-GRα showed no significant changes. We defined the first interaction as GC-GRα-driven, and the second interaction as NF-κB-driven [80]. The right panel shows non-improvers-survivors randomized after day 8 of ARDS to methylprednisolone (n = 11) vs. placebo (n = 6). After natural logarithmic transformation and adjustment for repeated measurements, partial correlations among responses to plasma from the methylprednisolone group were − 0.92 (p < 0.0001) both for nuclear NF-κB and nuclear GRα. For responses to plasma from the placebo group, no significant relationship was found between nuclear NF-κB and nuclear GRα (r = 0.11; p = 0.70) [7]. Prolonged methylprednisolone treatment was associated with upregulation in all measurements of GC-GRα-activity leading to reduction in NF-κB DNA-binding and transcription of inflammatory cytokines. Glucocorticoid treatment changed the longitudinal direction of systemic inflammation from dysregulated (NF-κB-driven, maladaptive response) to regulated (GRα-driven, adaptive response) with significant improvement in indices of alveolar-capillary membrane permeability and markers of inflammation, hemostasis, and tissue repair.
Reproduced with permission from reference [5]