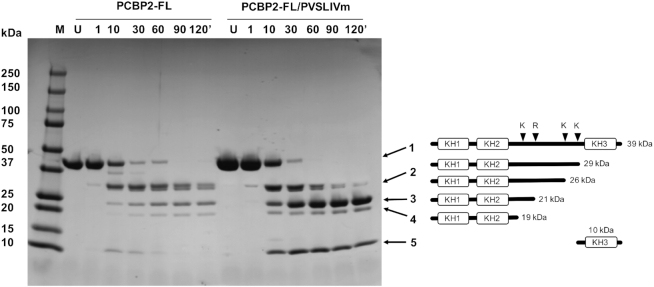
Figure 3.
Limited proteolysis of PCBP2-FL free and in complex with SLIVm. Susceptibility of PCBP2-FL to tryptic digest was monitored over time using SDS-PAGE (LHS). M = molecular mass standards; U = untreated protein; 1-120′ denotes the number of minutes of digestion by trypsin. The main bands were identified by N-terminal sequencing. Bands 1 to 4 were all identified as N-terminal fragments and band 5 was identified as KH3. The schematic (RHS) depicts the positions of lysine and arginine residues in the linker region of the full-length PCBP2. Also shown are representations of forms of PCBP2 cleaved at these four sites and their expected molecular masses. These correlate with the apparent masses of the tryptic digest cleavage products in the SDS-PAGE gel. These same bands were seen both in the absence and presence of bound SLIVm. Together this indicates that the PCBP2 linker region is not protected from proteases when PCBP2 is bound to SLIVm.