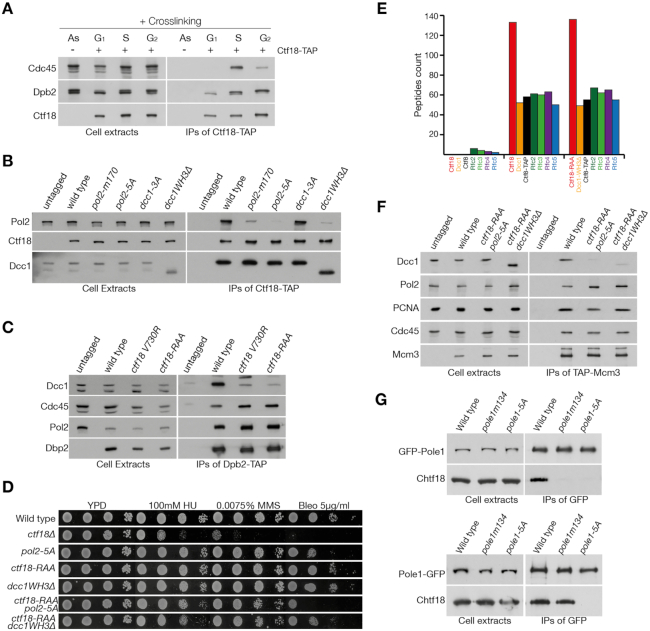
Figure 4.
Ctf18-RFC recruitment at forks depends on its binding to Pol2. (A) Analysis of the interaction of Ctf18-RFC and Pol ϵ in vivo. Cells, carrying a TAP-tagged or untagged version of CTF18 were grown to exponential phase (As) and arrested in G1, before being released into the cell cycle for 30 (S) or 60 min (G2). All cultures were treated with formaldehyde. The cross-linked protein extracts were then incubated with anti-TAP beads. Cell extracts and IPs were then analyzed by immunoblotting (B, C) Analysis of mutations in POL2, CTF18 and DCC1 on the Pol ϵ/Ctf18-RFC interaction. All mutants were generated at the genomic locus. These are pol2 170-GRAAAATGDAAG-181 (pol2 m170), pol2 330-AAIAAFA-336 (pol2-5A), dcc1 K364A, K367A, K380A (dcc1-3A) and dcc1(1–318) (dcc1WH3Δ). pol2-m170 was selected for analysis following a screen by Yeast-Two-Hybrids of conserved amino acids predicted to be on the surface of the protein (Supplementary Figure S4B-C). (B) Strains carrying a TAP-tagged allele of Ctf18, or an untagged control, were grown to exponential phase. Cell extracts and proteins immunoprecipitated using anti-TAP beads were analyzed by immunoblotting. (C) Wild type, ctf18 V730R, or ctf18 730-RAA-732 (ctf18-RAA) strains, carrying a DPB2 or a DPB2-TAP allele, were analyzed as above. (D) Combination of double mutations causes replication stress sensitivity. The indicated strains were diluted 1:10 and spotted on the specified medium. (E) Mass spectrometry analysis of the Ctf18-RFC complex in wild type and ctf18-RAA dcc1WH3Δ. Cells carrying a TAP-tagged or untagged allele of CTF8 were grown to the exponential phase before collection. Cell extracts were incubated with TAP-beads and washed at high salt (300 mM potassium acetate) before being analyzed by mass spectrometry. The number of peptides identified for the CTF18-RFC complex are shown. (F) Loss of interaction with Pol2 causes the displacement of Ctf18-RFC from replication forks. Cells carrying a TAP-tagged allele of MCM3 and an untagged control were synchronously released in S phase for 30 min and treated with formaldehyde. Cells extracts and the proteins immunoprecipitated with anti-TAP beads were analyzed by immunoblotting. (G) The interaction surface for the interaction between Pol ϵ and Ctf18-1-8 is conserved in human cells. HeLa cells were transiently transformed with plasmid expressing a N-terminal (top) or C-terminal (bottom) GFP-tagged alleles of POLE1, either wild type, pole1 134-GPAAAADGPAAG-145 (pole1-m134, modeled on the mutant pol2 m170), or pole1 315-AAIAAFA-321 (pole1-5A). Cells extracts were incubated with anti-GFP beads, immunoprecipitated and analyzed by immunoblotting.