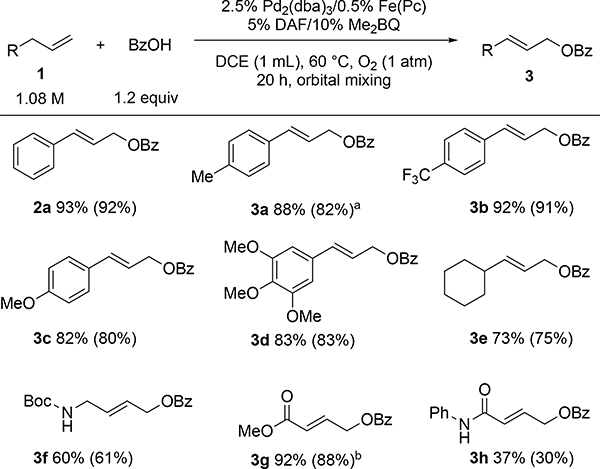
Figure 5.
Substrate scope. NMR % yield relative to 1,3,5-trimethoxybenzene (isolated % yield in parentheses). Average of two reactions. [a] Isolated yield on 1 g scale: 80% [b] Yields obtained using t-butylbenzoquinone due to co-elution with 2,6-dimethylbenzoquinone during column chromatography purification.