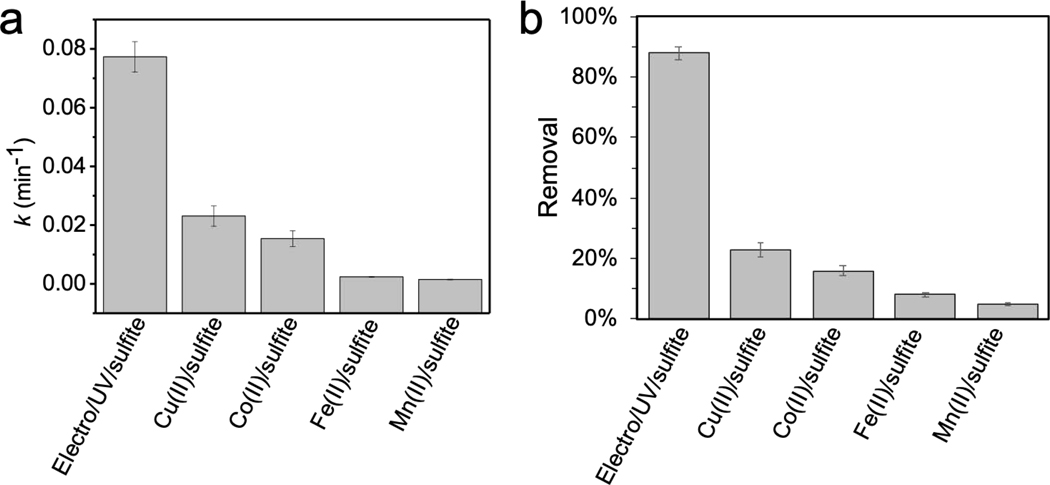
Fig. 5.
Comparisons of benzoic acid degradation in terms of (a) kinetics and (b) removals after reaction for 30 min by electro/UV/sulfite and metal/sulfite processes. Conditions: electro/UV/sulfite process − 5 μM benzoic acid, 1 mM sulfite, 10 mM Na2SO4, 200 mA, UV; metal/sulfite processes − 5 μM benzoic acid, 1 mM sulfite, 0.1 mM metal ions (i.e., Cu(II), Co(II), Fe(II), or Mn(II)). Reactions were carried out in 10 mM MOPS buffer at pH 7.