| 1 |
icas#3 |
(R)−8-(((2R,3R,5R,6S)−5-((1H-indole-3-carbonyl)oxy)−3-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)nonanoic acid |
Previously identified via synthesis (Srinivasan et al., 2012) |

|
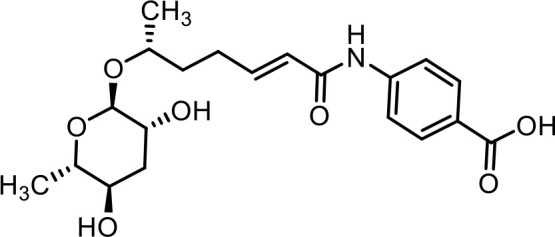
| 2 |
ascr#8 |
4-((R,E)−6-(((2R,3R,5R,6S)−3,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)hept-2-enamido)benzoic acid |
Previously identified via synthesis (Pungaliya et al., 2009) |
|
| 3 |
uglas#11 |
(2R,3R,4S,5R,6R)−5-hydroxy-6-(hydroxymethyl)−4-(phosphonooxy)−2-(2,6,8-trioxo-1,2,6,7,8,9-hexahydro-3H-purin-3-yl)tetrahydro-2H-pyran-3-yl (R)−6-(((2R,3R,5R,6S)−3,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)heptanoate |
Previously identified via synthesis (Curtis et al., 2020) |

|
| 4 |
ubas#3 |
(R)−4-(((2R,3R,5R,6S)−3-hydroxy-6-methyl-5-(((R)−2-methyl-3-ureidopropanoyl)oxy)tetrahydro-2H-pyran-2-yl)oxy)pentanoic acid |
Previously inferred via tandem mass spectrometry (Falcke et al., 2018) |

|
| 5 |
ascr#1 |
(R)−6-(((2R,3R,5R,6S)−3,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)heptanoic acid |
Previously identified via NMR and synthesis (Jeong et al., 2005) |

|
| 6 |
gluric#1 |
3-((2R,3R,4S,5S,6R)−3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)−7,9-dihydro-1H-purine-2,6,8 (3H)-trione |
Previously identified via synthesis (Curtis et al., 2020) |

|
| 7 |
ascr#7 |
(R,E)−6-(((2R,3R,5R,6S)−3,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)hept-2-enoic acid |
Previously identified via synthesis (Pungaliya et al., 2009) |

|
| 8 |
PABA |
4-Aminobenzoic acid |
Commercial product (Sigma-Aldrich) |

|
| 9 |
ascr#3 |
(R,E)−8-(((2R,3R,5R,6S)−3,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)non-2-enoic acid |
Previously identified via synthesis (Butcher et al., 2007) |

|
| 10 |
ascr#10 |
(R)−8-(((2R,3R,5R,6S)−3,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)nonanoic acid |
Previously identified via synthesis (Srinivasan et al., 2012) |

|
| 11 |
|
1H-indole-3-carboxylic acid |
Commercial product (Sigma-Aldrich) |

|
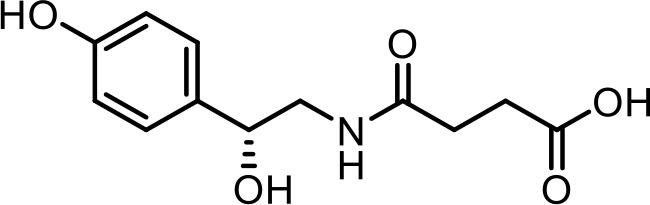
| 12 |
|
(R)−4-((2-hydroxy-2-(4-hydroxyphenyl)ethyl)amino)−4-oxobutanoic acid |
Identified via synthesis (This manuscript) |
|
| 13 |
iglas#1 |
((2R,3S,4S,5R,6R)−3,4,5-trihydroxy-6-(1H-indol-1-yl)tetrahydro-2H-pyran-2-yl)methyl (R)−6-(((2R,3R,5R,6S)−3,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)heptanoate |
Previously identified via synthesis (Artyukhin et al., 2018) |

|
| 14 |
glas#10 |
(2S,3R,4S,5S,6R)−3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl (R)−8-(((2R,3R,5R,6S)−3,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)nonanoate |
Previously identified via NMR and synthesis (Coburn et al., 2013) |

|
| 15 |
iglu#1 |
(2R,3S,4S,5R,6R)−2-(hydroxymethyl)−6-(1H-indol-1-yl)tetrahydro-2H-pyran-3,4,5-triol |
Previously identified via NMR and synthesis (Coburn et al., 2013) |

|
| 16 |
iglu#2 |
(2R,3R,4S,5R,6R)−3,5-dihydroxy-2-(hydroxymethyl)−6-(1H-indol-1-yl)tetrahydro-2H-pyran-4-yl dihydrogen phosphate |
Previously identified via NMR (Coburn et al., 2013) |

|
| 17 |
angl#1 |
(2S,3R,4S,5S,6R)−3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl 2-aminobenzoate |
Previously identified via NMR and synthesis (Coburn et al., 2013) |

|
| 18 |
angl#2 |
(2S,3R,4S,5R,6R)−3,5-dihydroxy-6-(hydroxymethyl)−4-(phosphonooxy)tetrahydro-2H-pyran-2-yl 2-aminobenzoate |
Previously identified via NMR (Coburn et al., 2013) |

|
| 19 |
iglu#4 |
(2R,3R,4S,5R,6R)−3,5-dihydroxy-6-(1H-indol-1-yl)−4-(phosphonooxy)tetrahydro-2H-(pyran-2-yl)methyl 2-aminobenzoate |
Proposed structure, based on identification of non-phosphorylated derivative (34) via synthesis (This manuscript) |

|
| 20 |
iglu#6 |
((2R,3R,4S,5R,6R)−3,5-dihydroxy-6-(1H-indol-1-yl)−4-(phosphonooxy)tetrahydro-2H-pyran-2-yl)methyl nicotinate |
Proposed structure, based on identification of non-phosphorylated derivative (SI-2) via synthesis (This manuscript) |

|
| 21 |
iglu#8 |
((2R,3R,4S,5R,6R)−3,5-dihydroxy-6-(1H-indol-1-yl)−4-(phosphonooxy)tetrahydro-2H-pyran-2-yl)methyl (E)−2-methylbut-2-enoate |
Proposed structure, based on identification of non-phosphorylated derivative (SI-3) via synthesis (This manuscript) |

|
| 22 |
iglu#10 |
((2R,3R,4S,5R,6R)−3,5-dihydroxy-6-(1H-indol-1-yl)−4-(phosphonooxy)tetrahydro-2H-pyran-2-yl)methyl 1H-pyrrole-2-carboxylate |
Proposed structure, based on identification of non-phosphorylated derivative (SI-4) via synthesis (This manuscript) |

|
| 23 |
iglu#12 |
((2R,3R,4S,5R,6R)−3,5-dihydroxy-6-(1H-indol-1-yl)−4-(phosphonooxy)tetrahydro-2H-pyran-2-yl)methyl benzoate |
Proposed structure. Inferred via tandem mass spectrometry (This manuscript) |

|
| 24 |
iglu#41 |
(2R,3R,4S,5R,6R)−6-(((2-aminobenzoyl)oxy)methyl)−5-hydroxy-2-(1H-indol-1-yl)−4-(phosphonooxy)tetrahydro-2H-pyran-3-yl 1H-pyrrole-2-carboxylate |
Proposed structure. Inferred from iglu#3 (34) via tandem mass spectrometry (This manuscript) |

|
| 25 |
angl#4 |
((2R,3R,4S,5R,6S)−6-((2-aminobenzoyl)oxy)−3,5-dihydroxy-4-(phosphonooxy)tetrahydro-2H-pyran-2-yl)methyl 2-aminobenzoate |
Proposed structure. Inferred from angl#3 (SI 5) via tandem mass spectrometry (This manuscript) |

|
| 26 |
tyglu#4 |
((2R,3R,4S,5R,6R)−5-((2-aminobenzoyl)oxy)−3-hydroxy-6-((4-(2-aminoethyl)phenoxy)−4-(phosphonooxy)tetrahydro-2H-pyran-2-yl))methyl 2-aminobenzoate |
Proposed structure. Initially described (O'Donnell et al., 2020) and further inferred via tandem mass spectrometry (This manuscript) |

|
| 27 |
ascr#81 |
(4-((R,E)−6-(((2R,3R,5R,6S)−3,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)hept-2-enamido)benzoyl)-L-glutamic acid |
Identified via synthesis (Artyukhin et al., 2018) |

|
| 28 |
ascr#82 |
((S)−4-carboxy-4-(4-((R,E)−6-(((2R,3R,5R,6S)−3,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)hept-2-enamido)benzamido)butanoyl)-L-glutamic acid |
Previously inferred via tandem mass spectrometry (Artyukhin et al., 2018) |

|
| 29 |
PABA-glu |
(4-aminobenzoyl)-L-glutamic acid |
Identified via synthesis (This manuscript) |

|
| 30 |
uglas#1 |
(2R,3R,4S,5S,6R)−4,5-dihydroxy-6-(hydroxymethyl)−2-(2,6,8-trioxo-1,2,6,7,8,9-hexahydro-3H-purin-3-yl)tetrahydro-2H-pyran-3-yl (R)−6-(((2R,3R,5R,6S)−3,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)heptanoate |
Identified via synthesis (Curtis et al., 2020) |

|
| 31 |
uglas#14 |
((2R,3S,4S,5R,6R)−3,4,5-trihydroxy-6-(2,6,8-trioxo-1,2,6,7,8,9-hexahydro-3H-purin-3-yl)tetrahydro-2H-pyran-2-yl)methyl (R)−6-(((2R,3R,5R,6S)−3,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)heptanoate |
Identified via synthesis (Curtis et al., 2020) |

|
| 32 |
uglas#15 |
((2R,3R,4S,5R,6R)−3,5-dihydroxy-4-(phosphonooxy)−6-(2,6,8-trioxo-1,2,6,7,8,9-hexahydro-3H-purin-3-yl)tetrahydro-2H-pyran-2-yl)methyl (R)−6-(((2R,3R,5R,6S)−3,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)heptanoate |
Previously inferred via tandem mass spectrometry (Artyukhin et al., 2018; Curtis et al., 2020) |

|
| 33 |
|
2-Aminobenzoic acid |
Commercial product (Sigma-Aldrich) |

|
| 34 |
iglu#3 |
((2R,3S,4S,5R,6R)−3,4,5-trihydroxy-6-(1H-indol-1-yl)tetrahydro-2H-pyran-2-yl)methyl 2-aminobenzoate |
Identified via synthesis (This manuscript) |

|
| 35 |
icas#2 |
(2S,3R,5R,6R)−5-hydroxy-2-methyl-6-(((R)−5-oxohexan-2-yl)oxy)tetrahydro-2H-pyran-3-yl 1H-indole-3-carboxylate |
Identified via synthesis (Dong et al., 2016) |

|
| 36 |
icas#6.2 |
(2S,3R,5R,6R)−5-hydroxy-6-(((2R,5S)−5-hydroxyhexan-2-yl)oxy)−2-methyltetrahydro-2H-pyran-3-yl 1H-indole-3-carboxylate |
Identified via synthesis (Dong et al., 2016) |

|
| SI 1 |
|
2-((tert-butoxycarbonyl)-amino)benzoic acid |
Characterized via synthesis (This manuscript) |

|
| SI 2 |
iglu#5 |
((2R,3S,4S,5R,6R)−3,4,5-trihydroxy-6-(1H-indol-1-yl)tetrahydro-2H-pyran-2-yl)methyl nicotinate |
Identified via synthesis (This manuscript) |

|
| SI 3 |
iglu#7 |
((2R,3S,4S,5R,6R)−3,4,5-trihydroxy-6-(1H-indol-1-yl)tetrahydro-2H-pyran-2-yl)methyl (E)−2-methylbut-2-enoate |
Identified via synthesis (This manuscript) |

|
| SI 4 |
iglu#9 |
((2R,3S,4S,5R,6R)−3,4,5-trihydroxy-6-(1H-indol-1-yl)tetrahydro-2H-pyran-2-yl)methyl 1H-pyrrole-2-carboxylate |
Identified via synthesis (This manuscript) |

|
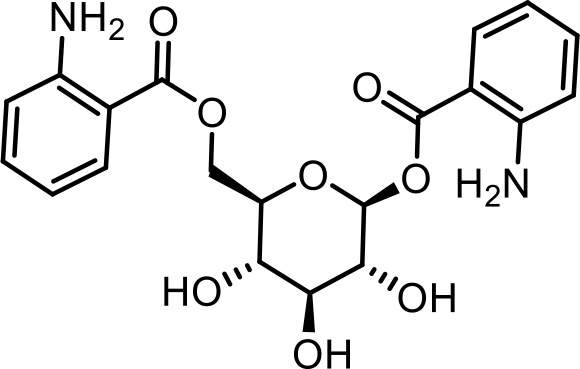
| SI 5 |
angl#3 |
((2R,3S,4S,5R,6S)−6-((2-aminobenzoyl)oxy)−3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)methyl 2-aminobenzoate |
Proposed structure based on synthesis of a reference sample for MS (This manuscript) |
|
| SI 6 |
tyglu#6 |
(2R,3R,4S,5S,6R)−6-(((2-aminobenzoyl)oxy)methyl)−2-((4-(2-aminoethyl)-phenoxy))−5-hydroxy-4-(phosphonooxy)-tetrahydro-2H-pyran-3-yl nicotinate |
Proposed structure. Initially described (O'Donnell et al., 2020) and further inferred via tandem mass spectrometry (This manuscript) |

|










































