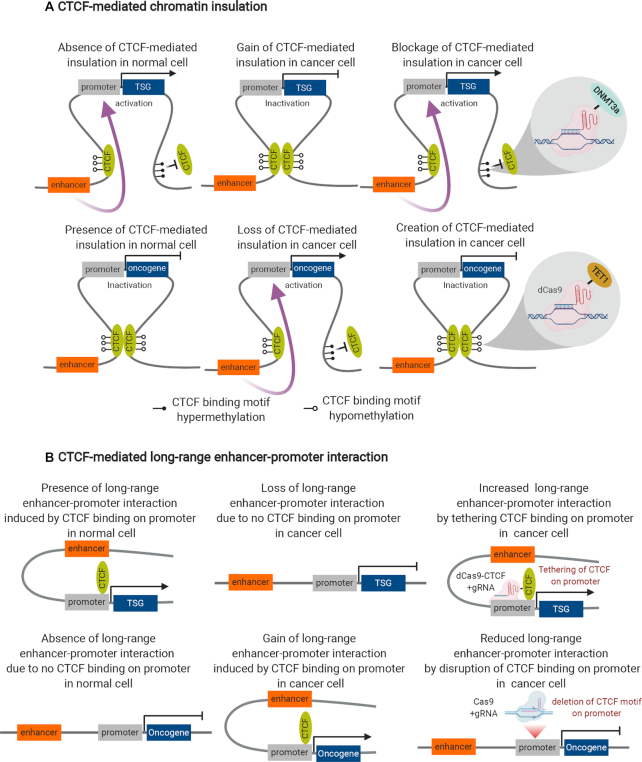
Figure 2.
Control of CTCF-mediated chromatin topology in cancer. (A) CTCF-mediated chromatin insulation: in a normal cell, DNA hypermethylation disrupts CTCF binding and looping, leading to abrogation of chromatin insulation, gain of enhancer–promoter interaction and transcription activation of tumour suppressor gene (TSG) or transcription repression of an oncogene. In a cancer cell, DNA hypomethylation facilitates CTCF binding and looping, leading to establishment of chromatin insulation, loss of enhancer–promoter interaction and transcription inactivation of tumour suppressor gene (TSG) or transcription activation of an oncogene. Epigenetic editing of CTCF-binding sites on the insulation anchors for TSG (dCas9-DNMT3a) and oncogene (dCas9-TET1) can lead to blockage or creation of CTCF insulation, gain or loss of enhancer–promoter interaction and transcription activation or inactivation for TSG and oncogene, respectively, in cancer cell. (B) CTCF-mediated long-range enhancer–promoter interaction: in a normal cell, CTCF binding on TSG promoter leads to establishment of enhancer–promoter interaction and transcription activation of TSG (vice versa for oncogene). In a cancer cell, loss of CTCF binding on TSG promoter leads to abrogation of enhancer–promoter interaction and transcription inactivation of TSG (vice versa for oncogene). Artificial tethering of CTCF on the TSG promoter via dCas9-gRNA approach can re-establish the enhancer–promoter interaction and transcription activation of TSG in cancer cell. Deletion of CTCF motif via Cas9-gRNA approach disrupts CTCF binding on the oncogene promoter, leading to abrogation of enhancer–promoter interaction and transcription inactivation of oncogene in cancer cells.