Figure 2.
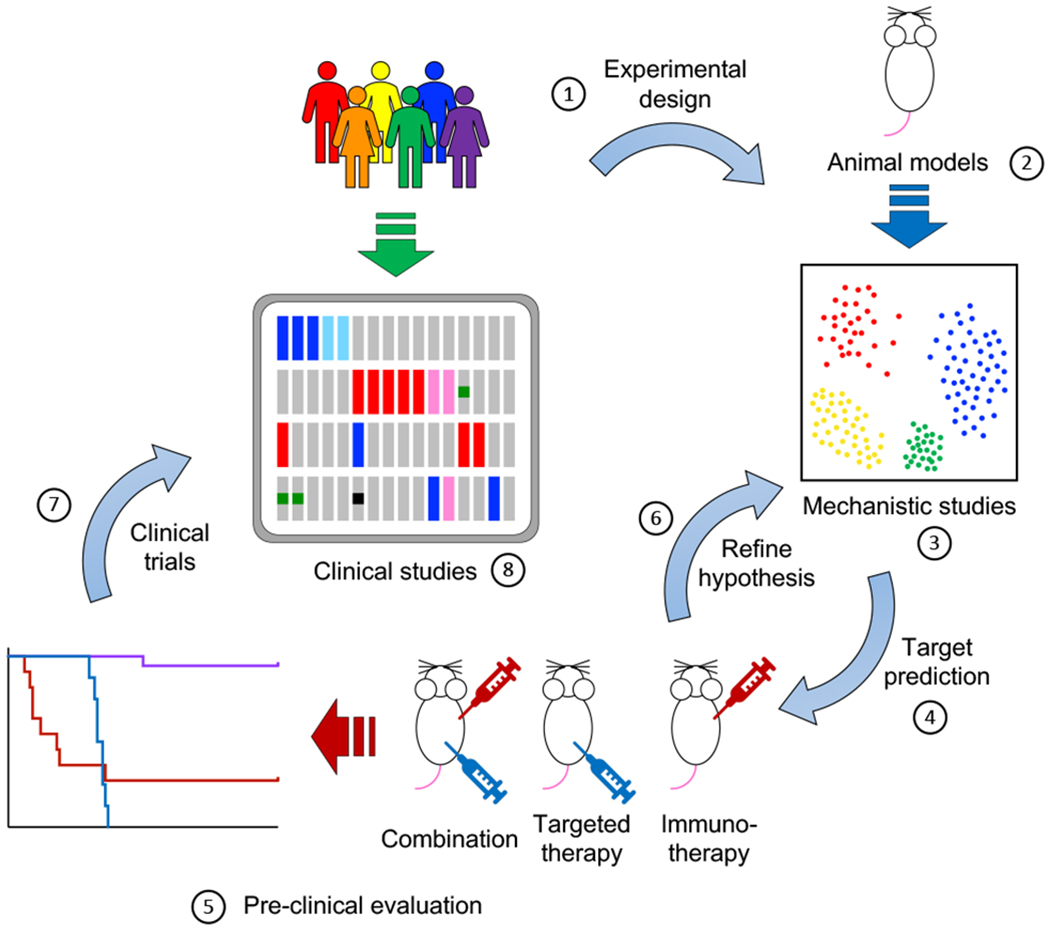
Integration of clinical and animal studies in translational immuno-oncology. (1) Experimental design informed by clinical observations to maximize translational potential. (2) Development of animal models that recapitulate genetic abnormalities found in the clinic. In this regard, immunocompetent, syngeneic mouse models provide the current gold standard. Emerging technologies, such as mouse models engrafted with humanized immune systems can improve the clinical relevance of pre-clinical studies and maximize translational feasibility. (3) Mechanistic studies are a crucial component of modern tumor immunology research. Complementing classical gain- and loss-of-function experiments, powerful technologies such as single-cell RNA sequencing (scRNA-Seq), cytometry by time-of-flight (CyTOF) and highly multiplex tissue cyclic immunofluorescence (t-CyCIF) have greatly enhanced our ability to interrogate the nature and degree of interplay between tumor and immune cells. Future work will undoubtedly entail more robust integration of single-cell expression analyses with single-cell spatial relationships within tissues. (4-6) Iterative rounds of target prediction (4), pre-clinical evaluation (5) and refining hypothesis (6) are needed to identify promising targets of clinical relevance. (7) Results from pre-clinical studies are used to inform and support the design of clinical trials for promising combinations.
