Figure 3. GRASP55 functions in unconventional protein secretion and autophagy.
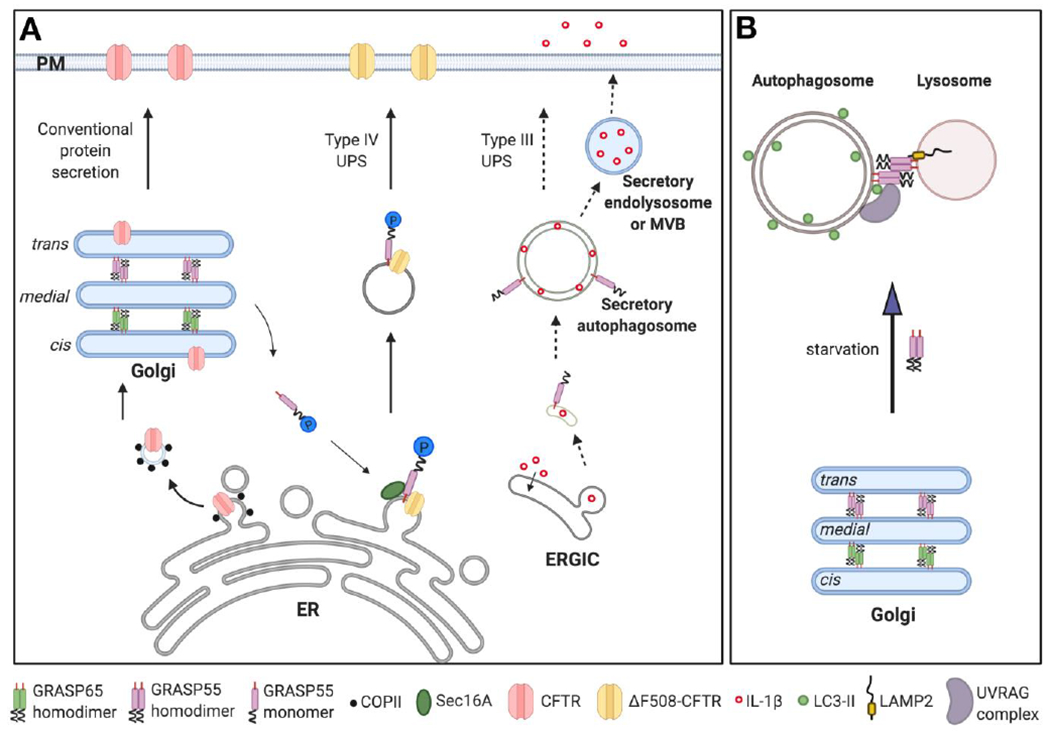
(A) Schematic model of GRASP55 function in unconventional protein secretion (UPS). Most transmembrane proteins, such as CFTR, are conventionally transported from the ER to the Golgi, and then to the plasma membrane (left, Conventional protein secretion pathway). Transmembrane ΔF508-CFTR can be delivered to the plasma membrane via a GRASP55-dependent and Golgi-independent pathway (middle, classified as Type IV UPS). ΔF508-CFTR UPS is induced by ER stress or inhibition of conventional protein secretion, which causes phosphorylation and monomerization of GRASP55. GRASP55 monomer then binds to ΔF508-CFTR and Sec16A at the ER exit sites and facilitates UPS. GRASP55 is also involved in unconventional secretion of some leaderless cytosolic proteins (right, classified as Type III UPS). IL-1β is one of the well-characterized cargo under starvation conditions. Once its mature form is produced, IL-1β is translocated into the ERGIC lumen, and then delivered by unconfirmed membrane carriers, possibly secretory autophagosomes, endolysosome or multi-vesicular bodies (MVB), to the plasma membrane for secretion. Here the exact role of GRASP55 is still unclear. (B) Schematic model of GRASP55 function in Golgi stacking and autophagosome-lysosome tethering. In growth conditions, GRASP55 localizes in medial-trans Golgi to glue adjacent cisternae together into stacks. Upon glucose- or amino acid-starvation, GRASP55 is translocated to the autophagosome and lysosome interface, where it binds LC3-II on autophagosomes and LAMP2 on lysosomes, and thereby facilitates the fusion of these two membrane organelles. Additionally, it promotes the targeting and assembly of the PtdIns3K UVRAG complex on autophagosomes to facilitate autophagosome maturation. For better visibility not all membranes are drawn on the same scale. Abbreviations: ER, Endoplasmic reticulum; ERGIC, the ER-Golgi intermediate compartment; MVB, multi-vesicular bodies; PM, plasma membrane; UPS, unconventional protein secretion.
