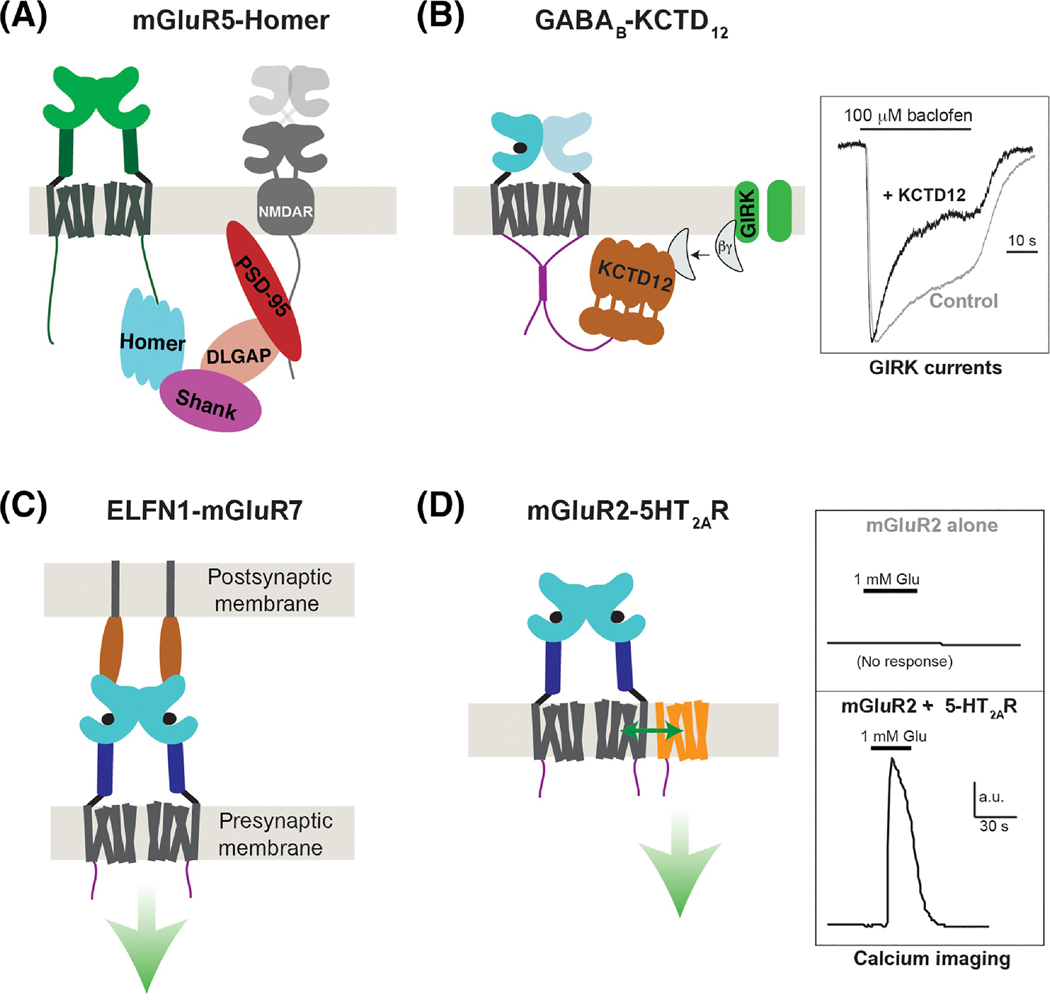
Figure 4, Modulation of class C GPCRs via accessory proteins.
A, Schematic showing group I mGluR (mGluR1/5) coupling to a network of intracellular scaffold proteins which control signaling within the post-synaptic density. In brief, mGluR1 or mGluR5 CTDs bind directly to Homer proteins which, via SHANK, DLGAP and PSD-95, facilitate cross-talk with NMDA-type ionotropic glutamate receptors. Homer has also been shown to facilitate coupling to IP3 receptors and other elements of the scaffold and trafficking machinery. B, Interaction between the GABAB2 CTD and KCTD12 enhances the activation kinetics and desensitization of agonist-induced GIRK potassium channel currents by binding Gβγ subunits (right). Note: crystal structures revealed that the KCTD12 BTB domain forms a ring structure that binds one CTD and the H1 domain forms a 5:5 pentameric complex with Gβγ to effectively scavenge the G proteins away from the channel. C, Trans-synaptic interactions between group III mGluRs and ELFN proteins control the synaptic localization of mGluRs and have been shown to allosterically modulate receptor activation properties (green arrow), although the effects on signaling are unclear. D, Heteromerization between mGluR2 and class A 5-HT2ARs enables various forms of functional crosstalk, including trans-activation of 5-HT2ARs to produce Gq signaling (i.e. calcium elevation) following mGluR2 agonism (right). The stability and stoichiometry of such heteromers are not clear but an interface involving the cytoplasmic end of TM4 has been demonstrated.