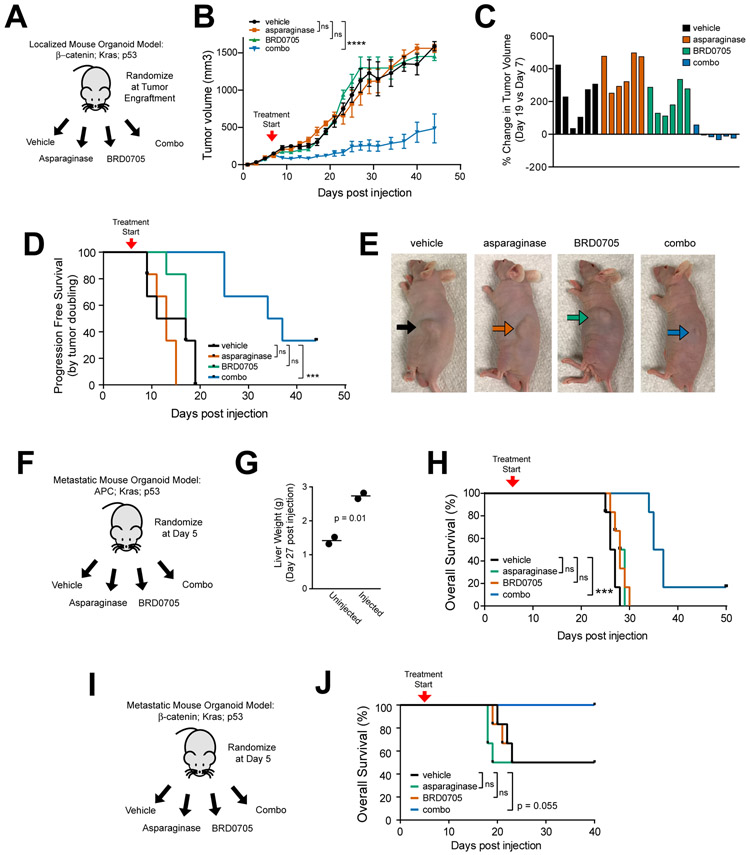
Figure 4. GSK3α Inhibition and Asparaginase for CRCs with APC or β-catenin Mutations.
A, Experimental schema. Triple-mutant mouse intestinal organoids with an activating β-catenin mutation, together with mutations of Kras and p53, were injected subcutaneously into male nude mice (n=6 per group). Once tumor engraftment was confirmed (>100 mm3 tumor volume), mice were randomized and treated with vehicle, asparaginase 1000 U/Kg x 1 dose, BRD0705 15 mg/kg every 12 hours x 21 days or both asparaginase and BRD0705 in combination (combo).
B, Tumor volumes of mice with β-catenin; Kras; p53 mutant tumors treated as in (A). Treatment start is denoted by the arrowhead on the graph. Significance was assessed by two-way ANOVA with Tukey adjustment for multiple comparisons, for tumor volume on day 19. Error bars represent SEM. **** p ≤ 0.0001. n.s., p > 0.05.
C, Waterfall plots showing % change in tumor volume over the first 14 days of treatment. Each bar represents an individual mouse.
D, Kaplan-Meier progression-free survival analysis of mice injected with indicated organoids and treated with vehicle or asparaginase from the experiment shown in (A). Progression-free survival was defined by time to death or doubling of tumor volume. Significance was assessed by log rank test. *** p ≤ 0.001. n.s., p > 0.05.
E, Representative images of anesthetized mice taken on day 14 post treatment from the experiment shown in (A). Arrows point to the location of the subcutaneous tumor.
F, Design of the experiment testing therapeutic activity in a liver-metastatic model of Apc; Kras; p53 triple-mutant mouse intestinal organoids. Treatment began on day 5 post-injection, and was performed as described in (A).
G, Liver weights of mice harvested to assess metastatic burden to the liver. Each data point represents an individual mouse. Significance was assessed by a two-sided Welch t-test.
H, Kaplan-Meier analysis of overall survival from mice in the experiment shown in (F) (n = 6 mice per group). Significance was assessed by log-rank test. *** p ≤ 0.001., *p ≤ 0.05 n.s., p > 0.05.
I, Design of the experiment testing therapeutic activity in a liver-metastatic model of β-catenin; Kras; p53 triple-mutant mouse intestinal organoids. Treatment began on day 5 post-injection, and was performed as described in (A).
J, Kaplan-Meier analysis of overall survival from mice in the experiment shown in (I) (n = 6 mice per group). Significance was assessed by log-rank test. *** p ≤ 0.001., *p ≤ 0.05 n.s., p > 0.05.