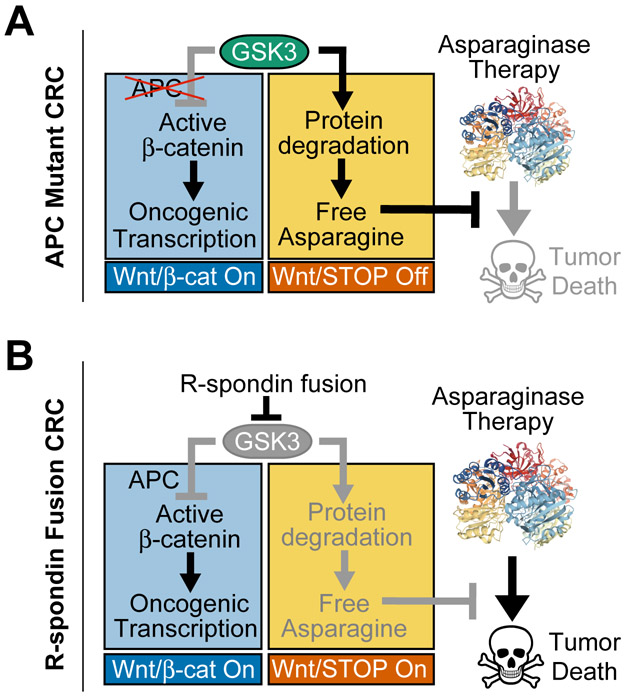
Figure 6. Model for Therapeutic Interaction of WNT Pathway Activation and Asparaginase.
A, Our model is that APC mutations selectively activate the β-catenin branch of WNT signaling downstream of GSK3. Thus, the ability of GSK3 to phosphorylate a large number of cellular proteins, leading to their ubiquitination and degradation, is unperturbed (WNT/STOP off). This degradation serves as a catabolic source of free asparagine, which prevents asparaginase-induced tumor death. Asparaginase structure is from PDB: 2HIM.
B, In R-spondin fusion CRC, WNT signaling is activated upstream of GSK3, which results in activation of both the WNT/β-catenin and the WNT-dependent stabilization of proteins (WNT/STOP) branches of this pathway. Activation of WNT/STOP inhibits protein degradation, limiting cellular asparagine availability, and triggering a unique vulnerability to its enzymatic degradation by asparaginase. Thus, asparaginase therapy induces tumor death in R-spondin fusion CRC. In APC-mutant CRC, selective inhibition of GSK3α phenocopies this effect.