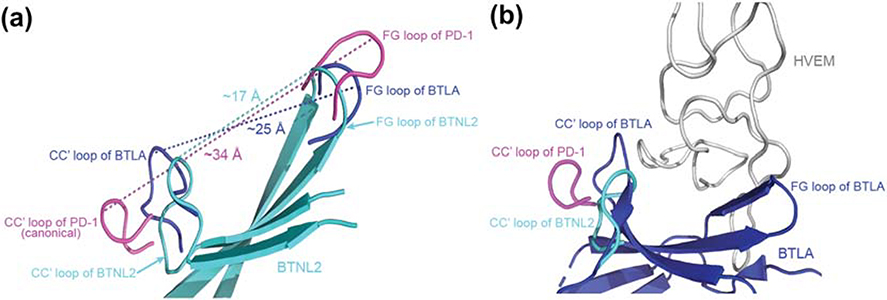
Figure 7: Non-canonical CC′ loop reduces the surface area of the front face of BTNL2.
A. Superimposed structures of BTNL2 (cyan), BTLA (blue) and PD-1 (magenta) depicts distances between their respective CC′ and FG loops. The CC′ and the FG loops (magenta) of PD-1 (a canonical IgV domain) flanks the front face binding interface and is separated by a distance of ~34Å. Contrastingly, the CC′ and FG loops (blue) of BTLA which binds to HVEM (a TNF receptor superfamily member) is separated by a distance of ~25Å. The CC′ and FG loops (cyan) of BTLN2 is even closer and is separated only by ~17Å. B. The CC′ loop of PD-1 flanks the interaction interface area of PD-1 which interacts with a wider IgV domain, whereas the CC′ loop of BTLA (blue) flanks the front face of BTLA, which in turn contributes to the binding of relatively narrow HVEM (grey). The CC′ loop of BTNL2 (cyan) appears to assume a similar conformation to that of BTLA, which could hint at a non IgSF binding partner whose interacting domain is of a similar size or even lower than HVEM. PDB files used are 2AW2 for BTLA and 3RRQ for PD-1.