Figure 1.
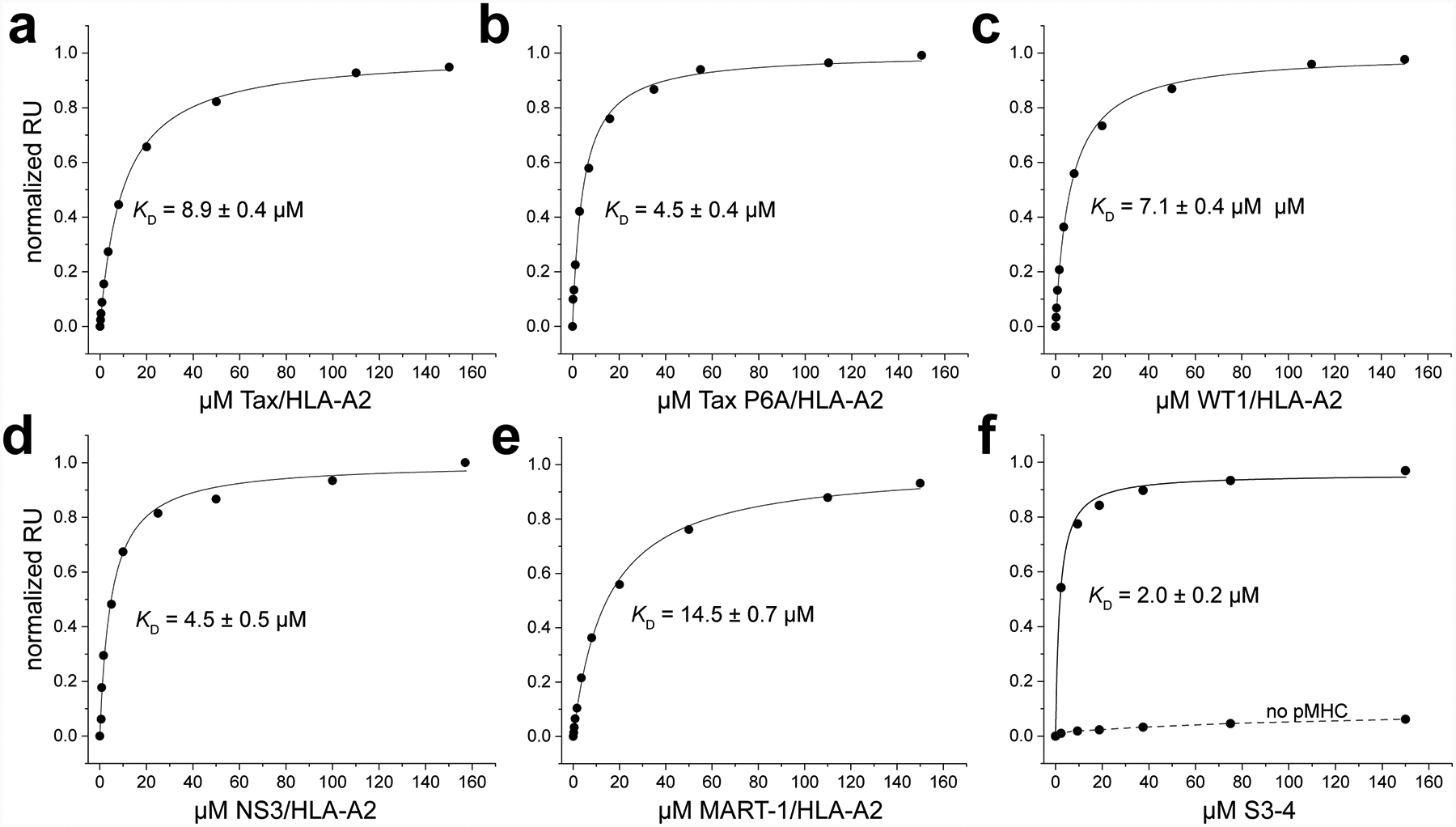
S3–4 binds peptide/HLA-A2 complexes independently of the bound peptide and does not compete for the HLA-A2 peptide binding groove. A-E) Equilibrium titrations with purified protein performed by SPR for different peptide/HLA-A2 complexes binding immobilized S3–4. Binding affinities and associated error are indicated in each inset. The results indicate relatively high affinity binding regardless of peptide, with the small variation resulting from an allosteric effect with different peptides. F) Blocking the peptide and peptide-binding domain of the Tax/HLA-A2 complex using the nM-binding high affinity c134 variant of the A6 TCR does not hinder binding of S3–4. Saturating amounts of Tax/HLA-A2 was injected over an A6 c134 surface to create a traditional TCR-peptide/HLA-A2 complex immobilized on the surface, followed by a normal titration of S3–4, which yielded a KD of 2 μM as indicated. The dashed line indicates responses measured without first injecting Tax/HLA-A2 over the A6 c134 surface, confirming that S3–4 binds the peptide/MHC.
