Figure 4.
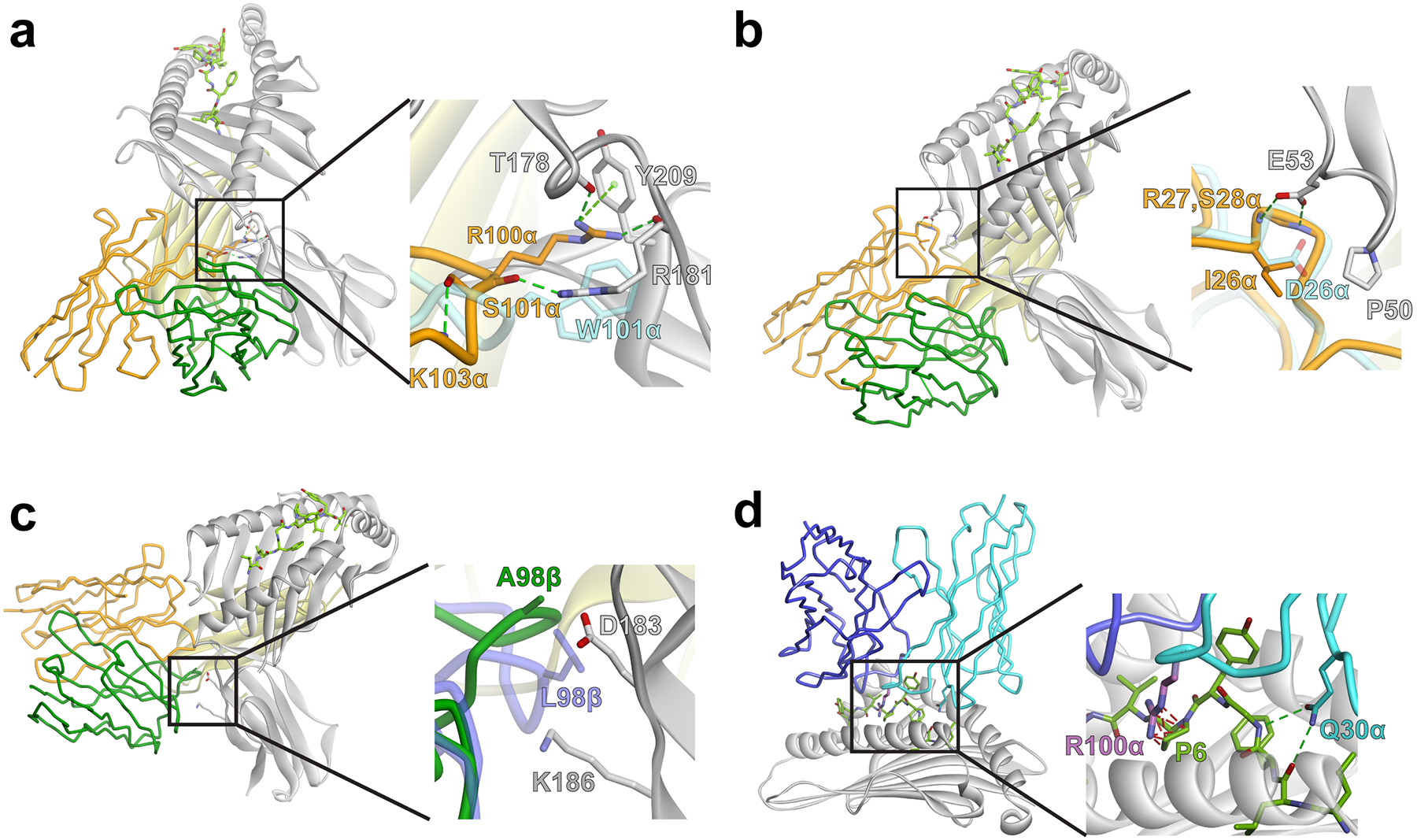
Deep mutational scanning of S3–4 demonstrates the collective importance of the six mutations in conferring peptide-independent binding. A) All single amino acid substitutions in the positions indicated were expressed in a yeast display library of S3–4. Mutants that bound with the top 1% fluorescence signal for MART-1/HLA-A2 at 5 nM were collected by FACS. Sequences were then analyzed using next-generation sequencing. The sequence enrichment compared with the naive library was calculated as a log2 ratio. Enrichment ratios are color-coded from ≤ 2−3 (orange) to ≥ 25 (blue). Stop codons are denoted with an asterisk. For the G102α residue, substitutions to T, N, Q, H, K, P, Y, W, I, L, M, Stop were completely depleted in the naïve library and therefore enrichment values could not be calculated; these residues are identified as negatively enriched (orange) in the scan. The cells boxed in red indicate those amino acids present in S3–4 and, for all positions except 99β, reflect the most frequently observed amino acid. The cells boxed in yellow indicate mutations incorporated to generate a higher affinity version of S3–4. B) Average enrichment values for all non-wild type substitutions were calculated and compared among S3–4, RD1-MART1, and the A6 c134 TCR (RD1-MART1 and A6 c134 enrichment values from previous data8). Error bars represent standard deviation of the average enrichment.
