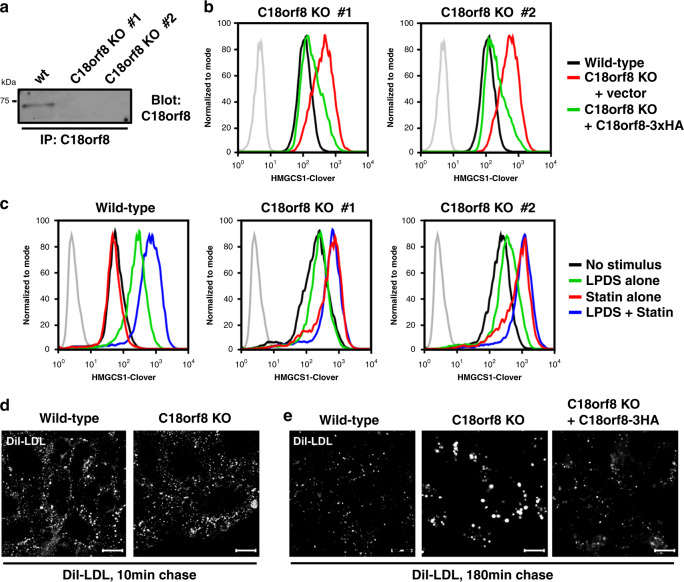
Fig. 3. C18orf8 is required for endosomal LDL-cholesterol uptake.
a, b C18orf8-deficient cells show spontaneous cholesterol deficiency. a Wild-type and C18orf8-deficient clones were lysed, endogenous C18orf8 immunoprecipitated, and detected by a C18orf8-specific antibody. b C18orf8-deficient HMGCS1-Clover clones were transduced with HA-tagged C18orf8 (C18orf8-3xHA; green lines) or empty vector (red lines) and HMGCS1-Clover expression was determined by flow cytometry at day 18 using wild-type HMGCS1-Clover cells as a control (black lines) (three independent experimental replicates). c C18orf8-deficient cells are dependent on endogenous cholesterol biosynthesis. Wild-type and C18orf8-deficient HMGCS1-Clover cells were either cultured in LPDS to block exogenous LDL-cholesterol uptake (green lines), treated with mevastatin to block endogenous cholesterol biosynthesis (red lines), or a combination of both treatments (blue lines), after which cells were analysed by flow cytometry for HMGCS1-Clover expression. d, e C18orf8-deficient cells show defective endo-lysosomal LDL degradation. Wild-type, C18orf8-deficient or C18orf8-deficient cells complemented with C18orf8-3xHA were starved for 1 h and pulse-labelled with fluorescent Dil-LDL, incubated for 5 min (d) or 180 min (e), fixed and visualised by confocal microscopy. Exposure times were kept constant between individual conditions at given time points. Representative images are shown from five fields per condition. Scale bars = 10 µm.